BELA: Bosutinib No Better Than Imatinib in Newly Diagnosed CML
Despite a better major molecular response rate, the Src/Abl tyrosine kinase inhibitor bosutinib did no better than imatinib with regard to complete cytogenetic response in the BELA trial of patients with newly diagnosed chronic myeloid leukemia (CML).
Despite a better major molecular response rate, the Src/Abl tyrosine kinase inhibitor bosutinib did no better than imatinib with regard to complete cytogenetic response in the BELA trial of patients with newly diagnosed chronic myeloid leukemia (CML).
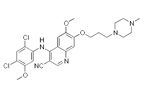
Chemical structure of bosutinib
Bosutinib (Bosulif) is approved for CML patients who have failed prior therapy. The BELA trial was a phase III study of 502 patients with newly diagnosed, chronic-phase CML; it was led by Jorge Cortes, MD, of the University of Texas MD Anderson Cancer Center in Houston, and published in the October 1 issue of the Journal of Clinical Oncology.
The complete cytogenetic response rate at 12 months was 70% among bosutinib patients and 68% among imatinib patients; this was a nonsignificant difference, meaning the primary endpoint of the trial was not met. However, 41% of bosutinib patients achieved a major molecular response at 12 months compared with 27% of imatinib patients (P < .001).
Furthermore, the median time to complete cytogenetic response was faster with bosutinib than with imatinib, at 12.9 weeks vs 24.6 weeks (P < .001). The complete molecular response at 12 months was 12% for bosutinib patients and 3% for imatinib patients (P < .001). Median time to major molecular response was also faster in the bosutinib patients than in the imatinib patients.
The authors wrote that the safety profiles for the two drugs were “distinct,” with gastrointestinal and live-related events more likely in bosutinib patients, and neutropenia and musculoskeletal disorders more common in the imatinib group. Adverse events causing treatment disruption occurred in 61% of bosutinib patients and 42% of imatinib patients, and dose reductions due to such events were necessary in 39% of bosutinib and 18% of imatinib patients. Serious events were more common in the bosutinib patients; grade 3 or 4 adverse events occurred in 64% of bosutinib patients and in 48% of imatinib patients (P < .001).
Cortes said in an e-mail to Cancer Network that in a setting of failed prior therapies bosutinib “offers a valuable new option for therapy. Based on its efficacy and good toxicity profile, it may help patients who have tried other therapies without a good response or with intolerance for such therapies.” The authors wrote that further follow-up is still needed to shed light on long-term outcomes including duration of response, transformation to accelerated-phase and blast-phase CML, and overall survival.
Study Details
Two patients in the bosutinib group and one in the imatinib arm did not end up receiving treatment, leaving 248 and 251 patients, respectively, for the safety analysis. Median ages in the two groups were 48 and 47 years, respectively; 60% of bosutinib patients and 54% of imatinib patients were male. The median time from diagnosis to study enrollment was about 22 days. All patients entered the study with either an ECOG performance status of 0 or 1.