
The Society for Immunotherapy of Cancer published clinical practice guidelines for treating patients with breast cancer using immunotherapy.
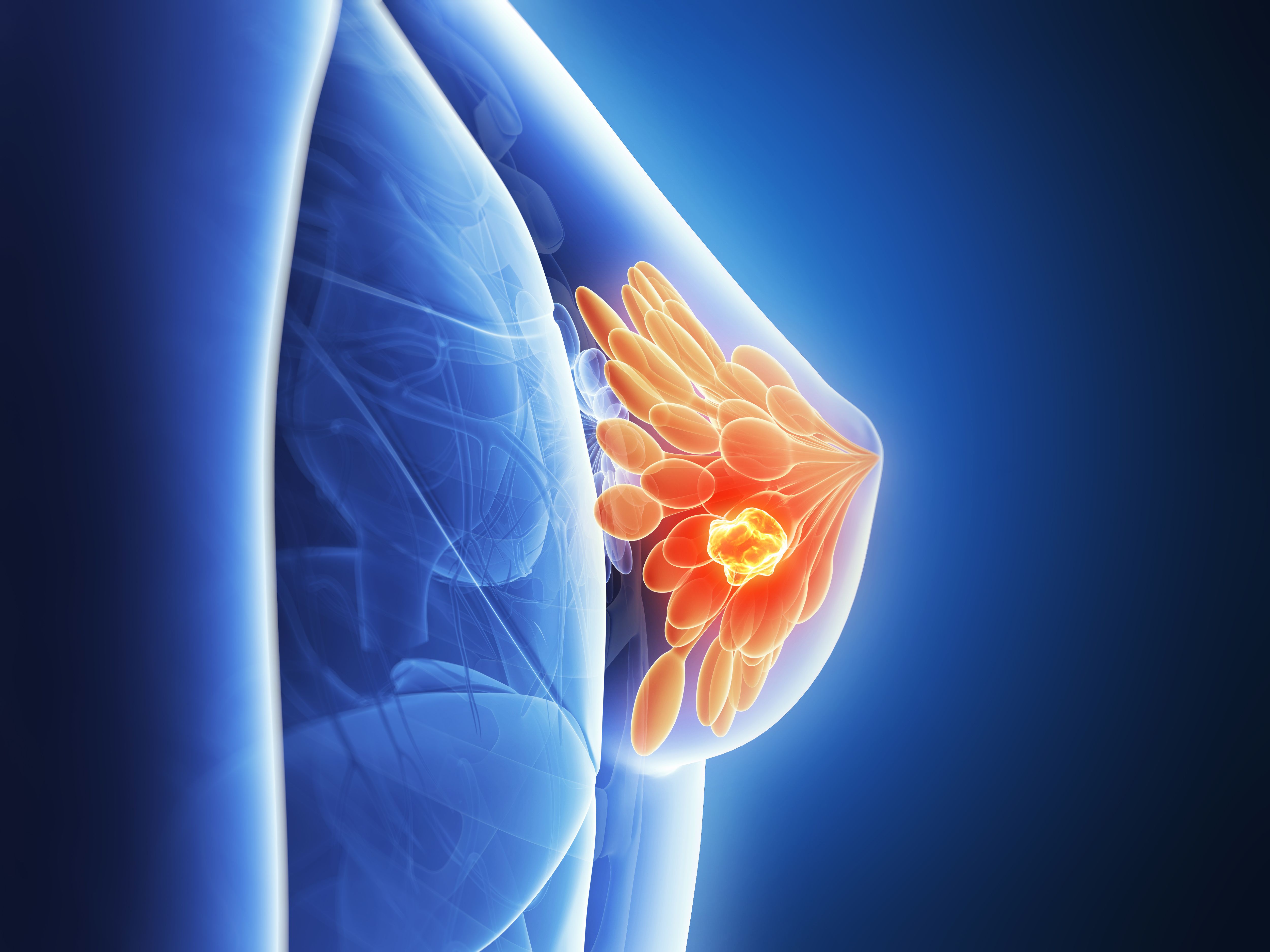
Approximately 270,000 women are diagnosed with breast cancer each year in the United States alone. While there is consensus among national organizations including the US Preventive Services Task Force, the American Cancer Society, and the American College of Radiology that routine mammography screening should be performed in women 50 years and older, there is debate about the benefit-to-harm ratio of routine screening in average-risk women aged between 40 and 49 years. In this review, we examine risks and benefits of routine breast cancer screening starting at age 40 at the individual level, followed by evaluation of the role of advanced imaging techniques in screening women on a population level.
Debu Tripathy, MD, of the University of Texas MD Anderson Cancer Center, comments on current NCCN recommendations for the use of prognostic and predictive gene profiling assays to individualize treatment in patients with HR-positive breast cancer.
A review of recent updates to genomic testing guidelines by the National Comprehensive Cancer Network and important takeaways that impact treatment decisions for HR+ breast cancer in the extended adjuvant setting.

A review of NCCN-recommended genomic assays and their impact on the management of HR+ breast cancer.
The Breast Cancer Index assay is the only of its kind to be recommended in the National Comprehensive Cancer Network Guidelines for the treatment of breast cancer as being predictive of extended adjuvant endocrine therapy.