New Second-Line Option for Advanced Gastric Cancer
The results of a phase III randomized trial found that the anti-angiogenesis antibody ramucirumab improved survival in advanced, previously treated gastric cancer.
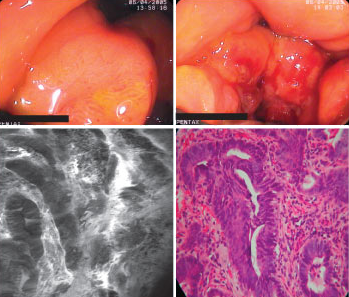
Advanced adenocarcinoma of the stomach. Top: conventional endoscopic views. Bottom: confocal endomicroscopic view (left) and histologic appearance of tumor tissue (right) showing good correspondence. Figures courtesy of Dr. Ralf Kiesslich, University of Mainz.
Results of a phase III randomized, international trial found that the anti-angiogenesis antibody ramucirumab is active as a second-line treatment in advanced gastric cancer. The drug showed an improvement in median survival of 1.4 months (5.2 months for the ramucirumab arm compared with 3.8 months for the placebo arm, P = .047). The results are published in the Lancet and were first presented at the American Association for Cancer Research (AACR) 2013 annual meeting. The results may be modest but notable considering the aggressiveness of gastric cancer and a lack of treatment options for patients with advanced disease.
Ramucirumab is the first anti-angiogenesis agent that has shown a survival benefit in gastric cancer.
The REGARD (Ramucirumab Monotherapy for Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma) trial randomized 355 patients 2:1 to either ramucirumab or placebo following disease progression with chemotherapy.
Bevacizumab, another VEGF inhibitor, has been tested in gastric cancer with limited results, but “the waning interest in VEGF as a therapeutic target in gastric cancer might now change on the basis of findings [from the REGARD trial],” wrote David H. Ilson, MD, of the Memorial Sloan-Kettering Cancer Center in New York, in an editorial of the results.
Ramucirumab also modestly improved progression-free survival by a median of 0.8 months (2.1 months in the experimental group vs 1.3 months in the placebo group, P < .0001).
The benefit of the antibody was similar to the survival benefit of second-line chemotherapy in gastric cancer but with a lower rate of toxic effects compared to chemotherapy, noted Ilson in the editorial. Ilson also stated that ramucirumab is likely to become the standard second-line therapy in advanced gastric cancer.
Adverse events related to treatment included abdominal pain, decreased appetite, fatigue, constipation, anemia, dysphagia, and vomiting. The rates of high-grade adverse events were similar between the two groups. Any-grade hypertension occurred in 16% of patients in the ramucirumab arm and in 8% of those in the placebo arm. Grade 3 or higher hypertension occurred in 8% and 3% of patients in the ramucirumab and placebo arms, respectively.
The trial reported limited quality of life (QoL) data on less than half of the patients in each arm as many patients in both arms discontinued treatment. Of the patients who did provide QoL data, a larger portion of patients in the ramucirumab arm had stable or improved quality of life compared with patients in the placebo arm. Of the 110 patients who provided QoL data in the experimental arm, 73% reported either stable or improved QoL, compared with 13% of patients on placebo who had stable or improved QoL. Because of the small sampling size, the difference was not statistically significant.
Worldwide, gastric cancer is the fourth leading cause of cancer-related deaths. There have been few new advancements in treating gastric cancer, although new adjuvant chemotherapy and radiotherapy combinations along with surgery have boosted survival. The standard of care for metastatic gastric cancer has remained the combination of a platinum-based chemotherapy with a fluorinated pyrimidine for more than 30 years. Median overall survival for advanced gastric cancer patients following first-line chemotherapy is approximately 8 to 10 months.
Gastric cancer is often aggressive, progressing rapidly to extensive metastatic disease. Ilson noted in the editorial that the patient population in the REGARD trial may be a relatively more healthy population that is not reflective of most patients.
The phase III RAINBOW trial combining ramucirumab with paclitaxel in patients with gastric cancer is currently ongoing. The manufacturer of ramucirumab, Eli Lilly, recently reported that the trial met its primary and secondary endpoints of improved overall survival and progression-free survival. The full results will be reported at a future scientific meeting.
Eli Lilly plans to seek approval for ramucirumab from the US Food and Drug Administration and European authorities based on the REGARD and the RAINBOW trials, according to a statement from the company.