Correct Answers:
1: C
2: B
A 55-year-old perimenopausal woman presented with a palpable lump in her left breast. Diagnostic mammogram showed a 1.8-cm spiculated mass with scattered microcalcifications within the mass. Comparison with her most recent prior mammogram (about 9 months earlier) showed this to be a new mass.
Oncology (Williston Park). 30(3):258–262.

Figure 1. Left Upper Outer Quadrant Spiculated Mass on Mammogram.
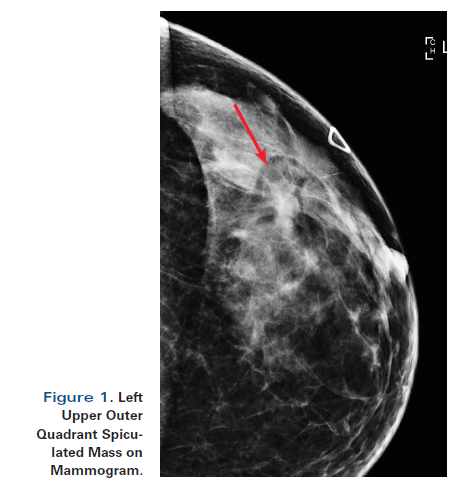
Figure 2. Same Lesion on Ultrasound.
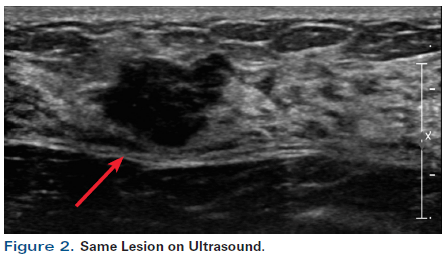
Figure 3. Same Lesion on MRI.
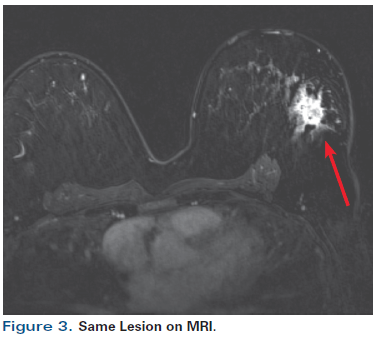
Figure 4. Prominent Internal Mammary Node.
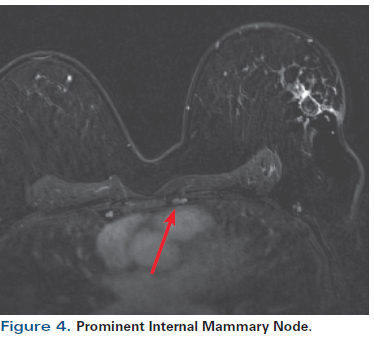
A 55-year-old perimenopausal woman presented with a palpable lump in her left breast. Diagnostic mammogram showed a 1.8-cm spiculated mass with scattered microcalcifications within the mass (Figure 1). Comparison with her most recent prior mammogram (about 9 months earlier) showed this to be a new mass. Ultrasound confirmed a hypoechoic mass of about 1.4 cm at the 2 o’ clock position in the left breast, 4 cm from the nipple (Figure 2). Axillary nodes appeared normal anatomically. A core biopsy was obtained, which demonstrated poorly differentiated invasive ductal carcinoma of at least 0.8 cm, associated with poorly differentiated ductal carcinoma in situ (DCIS). Estrogen receptor was 1+ in 1% (negative), progesterone receptor was negative, and human epidermal growth factor receptor 2 (HER2) was 0 by immunohistochemistry. A biopsy clip was placed. A magnetic resonance imaging (MRI) scan of both breasts was obtained at an outside institution (Figure 3). This showed no additional lesions; however, the tumor area seen on MRI was estimated at 2.5 cm. A 4-mm ipsilateral internal mammary node was noted (Figure 4). The axilla seemed normal. The patient had no family history of cancer but did have a history of hypertension. She stated that she had been advised to have a bilateral mastectomy.
A. The MRI has upstaged the patient’s cancer, indicating that she has a cT2N0 stage II triple-negative breast cancer (TNBC); a positron emission tomography (PET)/computed tomography (CT) scan is strongly recommended to help stage the patient correctly.
B. The MRI has upstaged the patient’s cancer, indicating that she has a cT2N3 stage IIIB TNBC because of the detected internal mammary node.
C. The patient has a cT1cN0 TNBC. No further staging is required.
A. The patient should receive adjuvant chemotherapy based on surgical findings. She should have bilateral mastectomy.
B. The patient should receive adjuvant chemotherapy based on surgical findings. She may have a lumpectomy.
C. The patient should receive preoperative treatment with either dose-dense doxorubicin and cyclophosphamide followed by docetaxel (AC-T) or docetaxel, doxorubicin, and cyclophosphamide (TAC).
D. The patient should receive preoperative treatment with docetaxel/carboplatin weekly followed by dose-dense doxorubicin and cyclophosphamide (AC).
TNBC is characterized by an aggressive biology, and more often presents as a palpable mass in young women rather than as a screen-detected cancer. Although the vast majority of TNBCs are operable, changes in the management of the axilla and in radiation therapy to the breast are now influencing the choice of treatment modalities and the sequence in which these are used in the multimodality approach to nonmetastatic TNBC, with preoperative chemotherapy becoming an increasingly attractive approach to operable TNBC.
The role of MRI. With rare exceptions, MRI of the breast is not recommended for a clinical stage I invasive ductal carcinoma. It finds additional disease in the ipsilateral breast in 16% of cases, but a third of these findings are false positives.[1,2] Moreover, the false-positive findings on breast MRI frequently lead to additional biopsies and mastectomies that in retrospect would not have been necessary.[1,3]
We typically use MRI in three situations:
1) In patients who are interested in breast conservation, as it may be useful to characterize suspected multifocal disease.
2) In patients with a known genetic breast cancer risk or a strong family history, since they have a high risk of clinically unsuspected contralateral disease, and if invasive disease were found, they would undergo sentinel lymph node biopsy during a bilateral mastectomy procedure.
3) In patients with invasive lobular carcinoma of the breast, since the extent of these tumors is poorly defined on mammogram and ultrasound, although this indication is not currently included in the National Comprehensive Cancer Network (NCCN) guidelines.[4]
Although ultrasound frequently underestimates the size of a lesion, MRI typically overestimates the size and extent of a tumor, particularly when done post-biopsy. In this case, the MRI upstaged the patient from a cT1 to a cT2 tumor.
1: C
2: B
Significance of an internal mammary node. In general, the spatial resolution of CT and breast MRI is more than adequate to detect internal mammary nodes. However, there is some controversy regarding the implications of an internal mammary node detected on MRI, as seen in this patient. These nodes may be pathologic-particularly if the breast cancer is medial or central, and especially if axillary lymph nodes are involved.[5-9] The literature suggests that internal mammary nodes that are detectable by imaging may be pathologic (N3).[10] There are no clear size criteria for determining pathologic involvement,[11] although some small series suggest that nodes larger than 5 mm should be considered abnormal.[10] Nodes larger than 5 mm would be considered for post-mastectomy radiation therapy, for example. If an internal mammary node is PET-avid, then it is likely to be involved; however, PET has poor sensitivity for masses smaller than 8 mm.[12] In one recent study of screening MRI, almost half the subjects-who had no known breast cancer (all had Breast Imaging–Reporting and Data System [BI-RADS] category 1 mammograms)-had detectable internal mammary nodes (average size, 4.5 mm [range, 2–9 mm]).[13] A second series corroborated these findings.[14] Different institutions have differing policies on radiation therapy to internal mammary nodes detected on CT or MRI. In general, detectable nodes in medial tumors (especially a tumor in the right breast) and detectable nodes in patients who also have involved axillary nodes may need to be treated, particularly if no heart needs to be in the radiation field. Because this patient’s tumor was in the upper outer quadrant of the breast, drainage to the internal mammary node was unlikely (thus, Answer 1B is incorrect).
Systemic staging. According to NCCN guidelines, a PET/CT scan is not recommended for patients with stage I/II breast cancer because the likelihood of finding overt metastatic disease is less than 3%, and false positives can occur.[4] Thus, Answer 1A is incorrect. There is some controversy as to whether all patients undergoing neoadjuvant therapy should be staged. Systemic staging is not recommended for stage I/II breast cancer in general, and is only definitively recommended for stage IIIB/IV disease in that setting. However, patients receiving neoadjuvant therapy are only staged clinically and not pathologically. Therefore, we do recommend staging when planning neoadjuvant therapy, making Answer 1C correct. In addition, a pretreatment echocardiogram is recommended if doxorubicin is being considered.
Genetic testing. Because this patient was aged 60 or younger and had a TNBC, she met the NCCN guideline criteria for undergoing genetic screening for breast cancer risk.[11] The likelihood of finding a BRCA1 germline mutation was greater than 10%.[11] Surgical choices would clearly be influenced by such results. The value of a gene panel is becoming established. In general, there is excellent concordance of BRCA mutations among such panels (> 90%), and additional pathogenic variants in other genes are also detected (~4%).[15,16]
In general, randomized trials demonstrate similar disease-free survival (DFS) and overall survival (OS) in patients given the same treatment preoperatively compared with postoperatively.[17] Neoadjuvant chemotherapy is generally considered when tumors are greater than 2 cm and particularly if nodes are clinically positive.[4] The chief benefit of preoperative chemotherapy is a reduction in the size of the primary tumor relative to the breast size, enabling breast-conserving surgery. It may also facilitate surgical management of the axilla in patients with bulky adenopathy. In addition, neoadjuvant therapy provides an opportunity to observe clinical and pathologic response to systemic therapy. Pathologic complete response (pCR) or near-pCR is associated with improved DFS and OS, particularly in situations in which all treatment is given preoperatively. Thus, the correlation is tightest for TNBC, somewhat less so for HER2-positive breast cancer, and least tight for estrogen receptor–positive breast cancer,[18,19] although the magnitude of the influence of pCR on DFS and OS is debated.[20] The administration of neoadjuvant therapy creates time to obtain the results of genetic testing and time for the surgeon to coordinate with the plastic surgeon. Given the recent changes in axillary management, preoperative chemotherapy may clear the axillary nodes of disease, allowing for lesser surgery if nodes were clinically positive originally (Alliance A011202 trial; ClinicalTrials.gov identifier, NCT01901094) and/or less extensive radiation therapy (National Surgical Adjuvant Breast and Bowel Project B-51 trial; ClinicalTrials.gov identifier, NCT01872975). However, these concepts are still being tested in clinical trials. It is not yet known whether patients whose tumors progress on preoperative chemotherapy can benefit from a change in such therapy.
The principal risk of giving preoperative chemotherapy is overtreatment. In this case, the tumor size could conceivably have ranged from 1.4 cm to 2.5 cm, and part of the tumor was DCIS. If the patient had a 2.5-cm TNBC, most oncologists would treat with a third-generation regimen (such as dose-dense AC-T [± carboplatin]).[21] However, if the invasive tumor were only 1.5 cm, many oncologists would opt for docetaxel plus cyclophosphamide (TC) × 4 cycles, thus sparing the patient short-term toxicity and much of the long-term risk of heart failure or leukemia.[22] In this patient, due to the need for a “tie-breaker,” we were influenced by the physical examination findings and by her hypertension and heart function on echocardiogram. Her examination revealed a lump of less than 2 cm, and we thus recommended surgery first, making Answers 2C and 2D incorrect.
The patient was willing to undergo lumpectomy with sentinel lymph node biopsy, pending the results of BRCA testing. She had been under the impression that contralateral mastectomy would improve her prognosis, but if her BRCA test results turned out to be normal, that would not have been the case, making Answer 2A incorrect.[23]
The final invasive tumor size was 1.6 cm, with negative margins. Two sentinel lymph nodes were negative. Echocardiogram demonstrated mild left ventricular hypertrophy, normal wall motion, and a left ventricular ejection fraction of 55%. She received 4 cycles of TC. The BRCA test results came back normal, and she had a 4-week course of hypofractionated radiation therapy with a boost.[24,25]
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
E. David Crawford, MD, serves as Series Editor for Clinical Quandaries. Dr. Crawford is Professor of Surgery, Urology, and Radiation Oncology, and Head of the Section of Urologic Oncology at the University of Colorado School of Medicine; Chairman of the Prostate Conditions Education Council; and a member of ONCOLOGY's Editorial Board.
If you have a case that you feel has particular educational value, illustrating important points in diagnosis or treatment, you may send the concept to Dr. Crawford at david.crawford@ucdenver.edu for consideration for a future installment of Clinical Quandaries.
1. Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248-58.
2. Pilewskie M, King TA. Magnetic resonance imaging in patients with newly diagnosed breast cancer. Cancer. 2014;120:2080-9.
3. Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7:193-201.
4. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer. Version 2.2015. J Natl Compr Canc Netw. 2015;13:1475-85.
5. Urban JA, Marjani MA. Significance of internal mammary lymph node metastases in breast cancer. Am J Roentgenol Radium Ther Nucl Med. 1971;111:130-6.
6. Byrd DR, Dunnwald LK, Mankoff DA, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001;8:234-40.
7. Sugg SL, Ferguson DJ, Posner MC, Heimann R. Should internal mammary nodes be sampled in the sentinel lymph node era? Ann Surg Oncol. 2000;7:188-92.
8. Chatzopoulos SE, Behranwala KA, Jayia P, et al. Should internal mammary lymph node biopsy be a routine step in recurrent breast cancer? Report of three cases with negative axilla and positive internal mammary node. World J Oncol. 2011;2:311-3.
9. Egner JR. AJCC cancer staging manual. JAMA. 2010;304:1726-7.
10. Kinoshita T, Odagiri K, Andoh K, et al. Evaluation of small internal mammary lymph metastases in breast cancer by MRI. Radiat Med. 1999;17:189-93.
11. Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014;145:707-14.
12. Wang CL, Eissa MJ, Roger JV, et al. 18F-FDG PET/CT positive internal mammary lymph nodes: pathologic correlation by ultrasound-guided fine-needle aspiration and assessment of associated risk factors. AJR Am J Roentgenol. 2013;200:1138-44.
13. Mack M, Chetlen A, Liao J. Incidental internal mammary lymph nodes visualized on screening breast MRI. AJR Am J Roentgenol. 2015;205:209-14.
14. Ray KM, Munir R, Wisner DJ, et al. Internal mammary lymph nodes as incidental findings at screening breast MRI. Clin Imag. 2015;39:791-3.
15. Lincoln SE, Kobayashi Y, Anderson MJ, et al. Systematic comparison of traditional and multigene panel testing for hereditary breast and ovarian cancer genes in more than 1000 patients. J Mol Diagn. 2015;15:533-44.
16. Kapoor NS, Curcio LD, Blakemore CA, et al. Gene panel testing detects equal rates of pathogenic BRCA1/2 mutations and has a higher diagnostic yield compared to limited BRCA1/2 analysis alone in patients at risk for hereditary breast cancer. Ann Surg Oncol. 2015;22:3282-8.
17. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778-85.
18. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-72.
19. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414-22.
20. Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: meta-regression of 29 randomized prospective studies. J Clin Oncol. 2014;32:3883-91.
21. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
22. Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research trial 9735. J Clin Oncol. 2009;27:1177-83.
23. Lester-Coll NH, Lee JM, Gogineni K, et al. Benefits and risks of contralateral prophylactic mastectomy in women undergoing treatment for sporadic unilateral breast cancer: a decision analysis. Breast Cancer Res Treat. 2015;152:217-26.
24. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086-94.
25. Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-20.