An 84-Year-Old Woman With an Indolent B-Cell Lymphoma
An 84-year-old woman with a history of Graves disease, hyperlipidemia, and hypertension presented to her physician with progressive fatigue and palpable bilateral axillary lymphadenopathy.
Oncology (Williston Park). 30(8):705–712.

Figure 1. Bone Marrow Pathology as Revealed on Biopsy
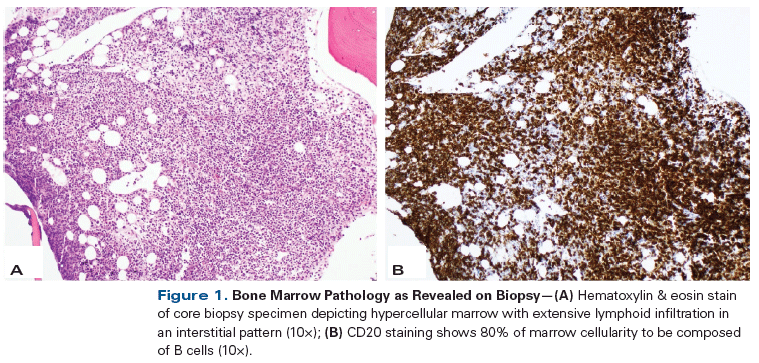
Figure 2. PET/CT Scan Obtained on Development of Progressive Neck Pain
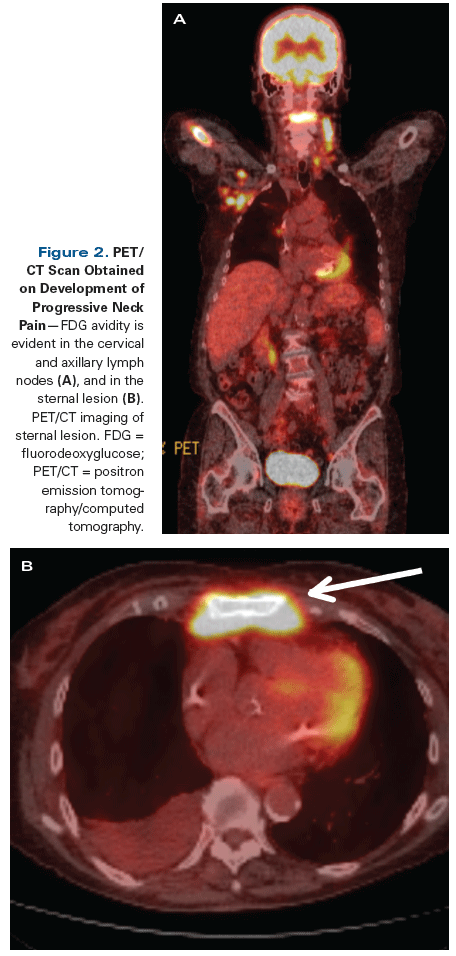
Table 1. NGS Analysis (405 genes) of Lymph Node

Figure 3. Lymph Node Pathology at Time of New Progressive Neck Pain

Table 2. Comparison of Characteristics of Selected Indolent B-Cell Non-Hodgkin Lymphomas

The Case
An 84-year-old woman with a history of Graves disease, hyperlipidemia, and hypertension presented to her physician with progressive fatigue and palpable bilateral axillary lymphadenopathy. Laboratory analysis revealed anemia (hemoglobin level of 9.5 g/dL) and mild thrombocytopenia (platelet count of 112,000/μL). A positron emission tomography (PET)/computed tomography (CT) scan was subsequently performed, which demonstrated a mildly fluorodeoxyglucose (FDG)-avid 3.8 × 2.3–cm mass in the right upper lobe. In addition, multiple prominent mediastinal and bilateral axillary lymph nodes were seen, although these did not demonstrate significant FDG uptake. Diffuse increased FDG uptake was also noted throughout the axial and appendicular skeleton, as well as in the spleen, raising concern for a lymphoproliferative disorder.
The patient underwent percutaneous CT-guided biopsy of her lung mass; immunohistochemistry (IHC) of the biopsy specimen showed atypical epithelioid cells positive for CK7, thyroid transcription factor 1, and napsin A, consistent with adenocarcinoma of the lung. A bone marrow biopsy was also performed; this revealed a hypercellular marrow with diffuse interstitial involvement by small atypical mature lymphocytes (Figure 1). IHC of the bone marrow specimen revealed an absence of cyclin D1 expression. Flow cytometry revealed a monotypic kappa-restricted CD5+ (partial), CD10−, and CD23− B-cell population. Cytogenetics were notable for monosomy X. Serum protein electrophoresis with immunofixation revealed an IgM kappa monoclonal protein level of 0.68 g/dL and a serum IgM level of 3,898 mg/dL.
The patient was determined to have stage I non–small-cell lung cancer (NSCLC) and an indolent B-cell non-Hodgkin lymphoma. Her NSCLC was treated with 5,200 cGy in 4 biweekly fractions, using stereotactic body radiotherapy with curative intent. It was felt that her B-cell non-Hodgkin lymphoma was most consistent with small lymphocytic lymphoma (SLL) or marginal zone lymphoma (MZL), and she was started on high-dose methylprednisolone combined with rituximab for 2 cycles, followed by rituximab combined with bendamustine for 5 cycles. By the end of treatment, her bone marrow showed no evidence of disease and her IgM level was undetectable.
She did well for 2 years until progressive neck pain developed. PET/CT at that time revealed new diffuse hypermetabolic activity involving her lymph nodes, axial skeleton, and bone marrow (Figure 2). She underwent a lymph node excisional biopsy to rule out metastatic NSCLC. The biopsy specimen revealed small mature atypical lymphocytes that were kappa-restricted; IgM+; and CD5−, CD10−, and CD23− (Figure 3). Plasmacytic differentiation was appreciated, and there was no evidence of large-cell transformation.
She was treated with palliative radiation therapy to her cervical vertebrae, followed by single-agent obinutuzumab, which resulted in complete resolution of her lymphadenopathy. Due to the diagnostic uncertainty regarding her lymphoma, hybrid-capture–based next-generation sequencing (NGS; Foundation Medicine)[1] was performed. NGS revealed four alterations (Table 1), including MYD88 L265P and CXCR4 S342fs*6 mutations.
Which of the following lymphomas is most likely this patient’s diagnosis?
A. MZL
B. Lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM)
C. Mantle cell lymphoma (MCL)
D. Chronic lymphocytic leukemia (CLL)/SLL
Discussion
This case demonstrates the diagnostic difficulty clinicians often encounter in determining the correct indolent B-cell non-Hodgkin lymphoma subtype. Table 2 illustrates the distinctive morphologic, immunophenotypic, and genetic features in the differential diagnosis of indolent B-cell non-Hodgkin lymphoma.
MZL is often difficult to differentiate from LPL. Both subtypes of lymphoma have a similar morphology and immunophenotype (CD5−/CD10−/CD23−), and sometimes both are characterized by the presence of an IgM monoclonal protein.[2] However, MZL typically has chromosomal abnormalities, such as trisomy 3 or t(11;18), and it rarely has the combination of MYD88 and CXCR4 alterations, as seen in this patient.[3] MYD88 mutations have been reported in less than 5% of splenic MZLs.[4] Thus, Answer A is unlikely. In addition, most cases of MZL have IgM levels < 0.5 g/dL.
MCL is typically comprised of medium-sized atypical lymphocytes, and its immunophenotype is usually CD5+/CD10−/CD23−.[5] The majority of cases have t(11;14), which results in increased expression of cyclin D1. This patient lacked this translocation on cytogenetic testing, and IHC did not demonstrate cyclin D1 expression. MCL tends to behave more aggressively than LPL and MZL. Furthermore, MYD88 mutations are rarely, if ever, seen in MCL. Answer C is thus incorrect.
KEY POINTS
- Lymphoplasmacytic lymphoma (LPL) is an indolent B-cell lymphoma that results in the accumulation of lymphoplasmacytic cells in the bone marrow and lymph nodes. When these malignant cells secrete a detectable IgM monoclonal protein, the entity is referred to as Waldenström macroglobulinemia (WM).
- MYD88 mutations occur in
more than 90% of LPL/WM
cases, and CXCR4 mutations occur in approximately 35%
of cases. Testing for MYD88 mutations, either by polymerase chain reaction–based
assay or next-generation
sequencing, should be
considered when there is
uncertainty regarding the
diagnosis of LPL/WM. - The Bruton tyrosine kinase
inhibitor ibrutinib is now
approved by the US Food and Drug Administration to treat both newly diagnosed and
relapsed LPL/WM.
It was initially felt that this patient had CLL/SLL (Answer D), and she was treated for this entity. However, typically CLL/SLL has a CD5+/CD10−/CD23+ immunophenotype (whereas this patient was CD23−). In addition, CLL/SLL frequently harbors del(11q), trisomy 12, del(13q), and del(17p). IgM monoclonal proteins can be seen in CLL/SLL; however, this occurs far less commonly than in LPL. MYD88 alterations are found in less than 5% of cases of CLL/SLL,[6] so the detection of an MYD88 L265P mutation makes it unlikely that CLL/SLL is the correct diagnosis here.
The findings in this patient are most consistent with LPL/WM (Answer B). The combination of plasmacytoid-appearing B cells with a CD5−/CD10−/CD23− immunophenotype, IgM monoclonal protein, and an MYD88 L265P mutation confirms the diagnosis.
LPL is a B-cell lymphoma that results in the accumulation of lymphoplasmacytic cells in the bone marrow and lymph nodes.[7] When these malignant cells secrete a detectable IgM monoclonal protein, the entity is referred to as WM. LPL/WM can have a wide array of symptoms, either directly related to tissue infiltration by the neoplastic cells or as manifestations of a paraneoplastic phenomenon related to the secreted IgM monoclonal protein. Morbidities related to the IgM monoclonal protein include hyperviscosity syndrome, peripheral neuropathy, cryoglobulinemia, cold agglutinin hemolytic anemia, and amyloidosis. Hyperviscosity syndrome, which results from the unique pentameric structure of IgM, can be life-threatening, and is usually treated with urgent plasmapheresis.[8] Patients with this syndrome can present with headaches, blurred vision, epistaxis, impaired mentation, and/or intracranial bleeding.
Over the past few years, significant advances have been made in the elucidation of key pathogenic alterations in LPL/WM. Recently, MYD88 and CXCR4 WHIM-like mutations have been found to be relatively specific for LPL/WM.[3,9-11] (WHIM-like mutations in CXCR4 were originally identified in the congenital immunodeficiency syndrome known as “warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis” [WHIM] syndrome.[12]) MYD88 mutations occur in more than 90% of cases, while CXCR4 mutations occur in approximately 35% of cases. Importantly, in one study, MYD88 L265P mutations were found in 58/58 (100%) of patients with WM, in 3/52 (4%) of patients with splenic MZL, and in 3/52 (4%) of patients with other B-cell chronic lymphoproliferative disorders, making this alteration both sensitive and specific for WM.[4] MYD88 mutations have also been shown to predict clinical presentation and survival.[13]
MYD88, an adaptor protein for Toll-like receptor signaling, is important in lymphomagenesis and signals through Bruton tyrosine kinase (BTK).[14] Recently, novel therapeutics, including the proteasome inhibitor bortezomib and the BTK inhibitor ibrutinib, have been shown to be highly efficacious in treating WM.[15,16] In a phase II trial, ibrutinib was able to produce an overall response rate of 91% in a group of previously treated patients with WM, leading to its approval by the US Food and Drug Administration for the treatment of both newly diagnosed and relapsed disease.[16] Furthermore, the response rates seen with ibrutinib were significantly higher in patients with MYD88 mutations than in those with wild-type MYD88.[17] Plerixafor, a CXCR4 inhibitor, is currently approved for use in combination with granulocyte colony–stimulating factor to mobilize hematopoietic stem cells to peripheral blood for autologous stem cell transplantation in patients with non-Hodgkin lymphoma or multiple myeloma.[18] CXCR4 mutations are potentially targetable with this agent.[19]
This case highlights the importance of distinguishing LPL/WM from other indolent B-cell lymphomas, justifying the use of molecular testing for MYD88 mutations, especially when the diagnosis is in doubt. Although NGS was used in this patient, a polymerase chain reaction–based assay is widely available that can detect the MYD88 L265P mutation. However, this assay will miss less common MYD88 mutations, including the MYD88 S243N and MYD88 M232T variants, which can be identified on NGS.[17] For this patient, detection of the MYD88 L265P mutation greatly aided in the diagnosis, as well as identifying a targetable mutation. The identification of MYD88 alterations is critically important when there is difficulty in distinguishing between the diagnoses of LPL/WM and MZL. This patient was initially thought to have MZL or CLL/SLL. It was not until MYD88 L265P was identified that the diagnosis of LPL/WM was considered. Now that the correct entity has been diagnosed, the use of ibrutinib is justified at relapse.
Financial Disclosure:Dr. Goodman receives fellowship funding from Pfizer. Dr. Choi serves on the speakers bureau for Gilead and on the advisory board of (and as a consultant to) AbbVie and Pharmacyclics; he also receives research funds from AbbVie and Pharmacyclics. Dr. Kurzrock receives research funding from Foundation Medicine, Genentech, Guardant Health, Merck, Pfizer, Sequenom, and Serono; receives consultant fees from Actuate Therapeutics, Sequenom, and X Biotech; and has an ownership interest in CureMatch, Inc and Novena, Inc. Dr. Wang has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
E. David Crawford, MD, serves as Series Editor for Clinical Quandaries. Dr. Crawford is Professor of Surgery, Urology, and Radiation Oncology, and Head of the Section of Urologic Oncology at the University of Colorado School of Medicine; Chairman of the Prostate Conditions Education Council; and a member of ONCOLOGY's Editorial Board.
If you have a case that you feel has particular educational value, illustrating important points in diagnosis or treatment, you may send the concept to Dr. Crawford at david.crawford@ucdenver.edu for consideration for a future installment of Clinical Quandaries.
References:
1. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023-31.
2. Thieblemont C, Molina T, Davi F. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127:2064-71.
3. Xu L, Hunter ZR, Yang G, et al. MYD88 L265P in Waldenström macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood. 2013;121:2051-8.
4. Varettoni M, Arcaini L, Zibellini S, et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenström’s macroglobulinemia and related lymphoid neoplasms. Blood. 2013;121:2522-8.
5. Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125:48-55.
6. Jeromin S, Weissmann S, Haferlach C, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28:108-17.
7. Treon SP. How I treat Waldenström macroglobulinemia. Blood. 2015;126:721-32.
8. Stone MJ, Bogen SA. Evidence-based focused review of management of hyperviscosity syndrome. Blood. 2012;119:2205-8.
9. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367:826-33.
10. Hunter ZR, Xu L, Yang G, et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123:1637-46.
11. Poulain S, Roumier C, Doye E, et al. Genomic landscape of CXCR4 mutations in Waldenstrom’s macroglobulinemia. Blood. 2014;124:1627. http://www.bloodjournal.org/content/124/21/1627. Accessed July 27, 2016.
12. Wetzler M, Talpaz M, Kleinerman ES, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89:663-72.
13. Treon SP, Cao Y, Xu L, et al. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123:2791-6.
14. Rossi D. Role of MYD88 in lymphoplasmacytic lymphoma diagnosis and pathogenesis. Hematology Am Soc Hematol Educ Program. 2014;2014:113-8.
15. Dimopoulos MA, Garcia-Sanz R, Gavriatopoulou M, et al. Primary therapy of Waldenström macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122:3276-82.
16. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430-40.
17. Treon SP, Xu L, Hunter Z. MYD88 mutations and response to ibrutinib in Waldenström’s macroglobulinemia. N Engl J Med. 2015;373:584-6.
18. DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767-73.
19. Scala S. Molecular pathways: targeting the CXCR4-CXCL12 axis-untapped potential in the tumor microenvironment. Clin Cancer Res. 2015;21:4278-85.