A Review of the 2022 SABCS Twitter Takeover
During the 2022 San Antonio Breast Cancer Symposium, Neil M. Iyengar, MD, took over the CancerNetwork® Twitter to review key presentation takeaways.
Neil M. Iyengar, MD

The 2022 San Antonio Breast Cancer Symposium (SABCS)was designed to bring experts together and create a platform in which groundbreaking breast cancer research can be shared. Results from these presentations will then be used to inform treatment decisions in the real-world setting including potential treatment options for patients with ESR1-mutant estrogen receptor (ER)–positive/HER2-negative breast cancer and hormone receptor–positive advanced breast cancer.
During General Session 3 of the conference, CancerNetwork® hosted a Twitter Takeover featuring Neil M. Iyengar, MD, associate attending physician at Memorial Sloan Kettering Cancer Center in New York, New York. In a #CNRealtimeReport, Iyengar discussed these presentations and provided the key takeaways from each trial.
Phase 3 EMERALD Trial
The first trial Iyengar covered was the phase 3 EMERALD study (NCT03778931), which “supports elacestrant as monotherapy in 2nd/3rd line” for patients with ER-positive/HER2-negative breast cancer, Iyengar wrote during the takeover.
Phase 3 EMERALD Trial
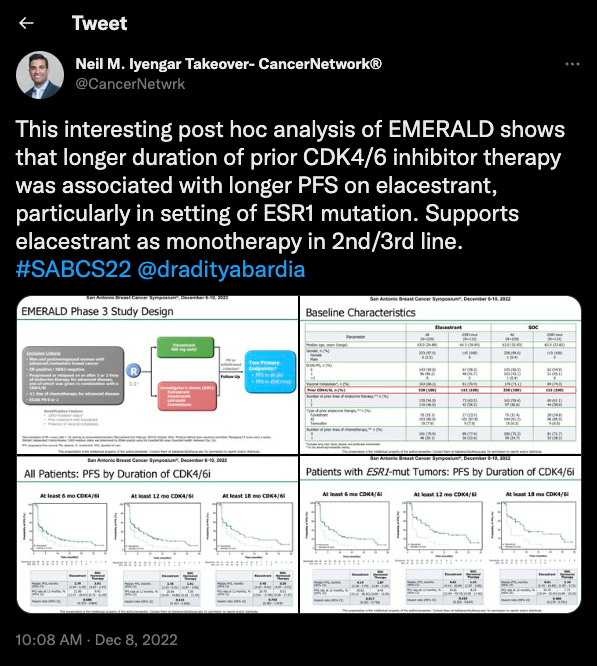
The trial included patients who had previously been treated with CDK4/6 inhibitors with a subgroup of patients with ESR1-mutant disease. Patients received treatment with either elacestrant or standard of care therapy (SOC).
A total of 87.5% of patients who completed a minimum of 6 months of treatment with a CDK4/6 therapy; among these patients, investigators reported a 31.2% increase in progression-free survival (PFS) vs SOC (HR, 0.688; 95% CI, 0.535-0.884). Additionally, the median PFS for patients in the this subgroup was 2.79 months (95% CI, 1.94-3.78) in the elacestrant arm and 1.91 months (95% CI, 1.87-2.14) in the SOC arm.
At 6 months, those with an ESR1 mutation had a median PFS of 4.14 months (95% CI, 2.20-7.79) in the elacestrant arm vs 1.87 months (95% CI, 1.87-3.29) in the SOC arm (HR, 0.517; 95% CI, 0.361-0.738).
A total of 3.4% of patients in the elacestrant arm and 0.9% of patients in the SOC arm needed to discontinue the trial due to treatment-related adverse effects.
Phase 2 SERENA Trial
Next, Iyengar touched on the significance of the phase 2 SERENA trial (NCT04214288) set out to determine the median PFS of 2 doses of camizestrant monotherapy vs SOC fulvestrant in patients with ER-positive/HER2-negative advanced breast cancer. “Benefit [was] seen in the overall population and ESR1 mutation [subgroup] at the 75 mg and 150 mg doses,” Iyengar wrote.
Phase 2 SERENA Trial
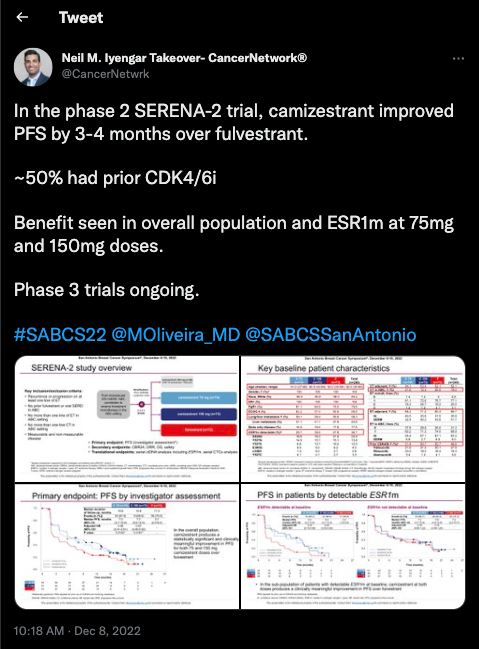
The trial consisted of multiple camizestrant cohorts with different doses including 300 mg, 75 mg, and 150 mg as well as a fulvestrant cohort. It was noted that the 300 mg cohort data has not been formally analyzed as the treatment arm ended early.
The median PFS in the 75 mg group was 7.2 months (95% CI, 3.7-10.9; HR, 0.58; 95% CI, 0.41-0.81; P = .0124), 7.7 months(95% CI, 5.5-12.9; HR, 0.67; 95% CI, 0.48-0.92; P = .0161) in the 150 mg cohort, and 3.7 months 95% CI, 2.0-6.0)in the fulvestrant cohort (.
On cycle 2, day 1, camizestrant was found to reduce ESR1-mutant ctDNA levels to undetectable or close to undetectable in the 75 mg and 150 mg treatment arms. Additionally, the overall response rate was 15.7% in the 75 mg cohort (OR, 1.43; 95% CI, 0.63-3.33; 2-sided P-value = .4789), 20.0% in the 150 mg cohort (OR, 1.96; 95% CI, 0.88-4.51; 2-sided P-value = .1675), and 11.8% in the fulvestrant cohort.
Phase 3 CAPItello-291 Trial
Data from the phase 3 CAPItello trial (NCT04305496) indicated that capivasertib plus fulvestrant vs placebo were found to improve PFS for patients with hormone receptor–positive/HER2-negative advanced breast cancer, including those with AKT pathway–altered tumors. Iyengar highlighted the regimen’s safety profile during the trial during his takeover, noting that diarrhea and rash were common AEs in the study.
Phase 3 CAPItello-291 Trial

In the capivasertib arm, the investigator-assessed PFS was 7.2 months (95% CI, 7.2-7.4) vs 3.6 months (95% CI, 2.8-3.7) in the placebo arm (HR, 0.60; 95% CI, 0.51-0.71; 2-sided P <.001). In those with AKT pathway mutations, the median PFS was 7.3 months (95% CI, 5.5-9.0) in the capivasertib arm vs 3.1 months (95% CI, 2.0-3.7) in the placebo arm (HR, 0.50; 95% CI, 0.38-0.65; 2-sided P <.001). Additionally, those in the non-altered population had a median PFS of 7.2 months (95% CI, 4.5-7.4) in the capivasertib arm vs 3.7 months (95% CI, 3.0-5.0) in the placebo arm (HR, 0.70; 95% CI, 0.56-0.88).
The planned OS analysis reached 28% maturity in the overall population (HR, 0.74; 95% CI, 0.56-0.98) and the AKT-altered pathway group (HR, 0.68; 95% CI, 0.45-1.05).
The grade 3 or higher AEs in the capivasertib vs placebo groups included diarrhea (9.3% vs 0.3%), maculopapular rash (6.2% vs 0.0%), rash (5.4% vs 0.3%), hyperglycemia (2.3% vs 0.3%), and stomatitis (2.0% vs 0.0%). In the AKT-altered group, the safety profile was similar.
Phase 2 PACE Trial
Finally, results from the phase 2 PACE trial (NCT03147287) were discussed at the meeting, as well as in Iyengar’s takeover. In the trial, palbociclib (Ibrance) and fulvestrant were found to not improve efficacy over fulvestrant alone for patients with ER–positive/HER2 negative breast cancer. Iyengar noted that the addition of the PD-L1 inhibitor avelumab (Bavencio) helped to prolong PFS.
Phase 2 PACE Trial
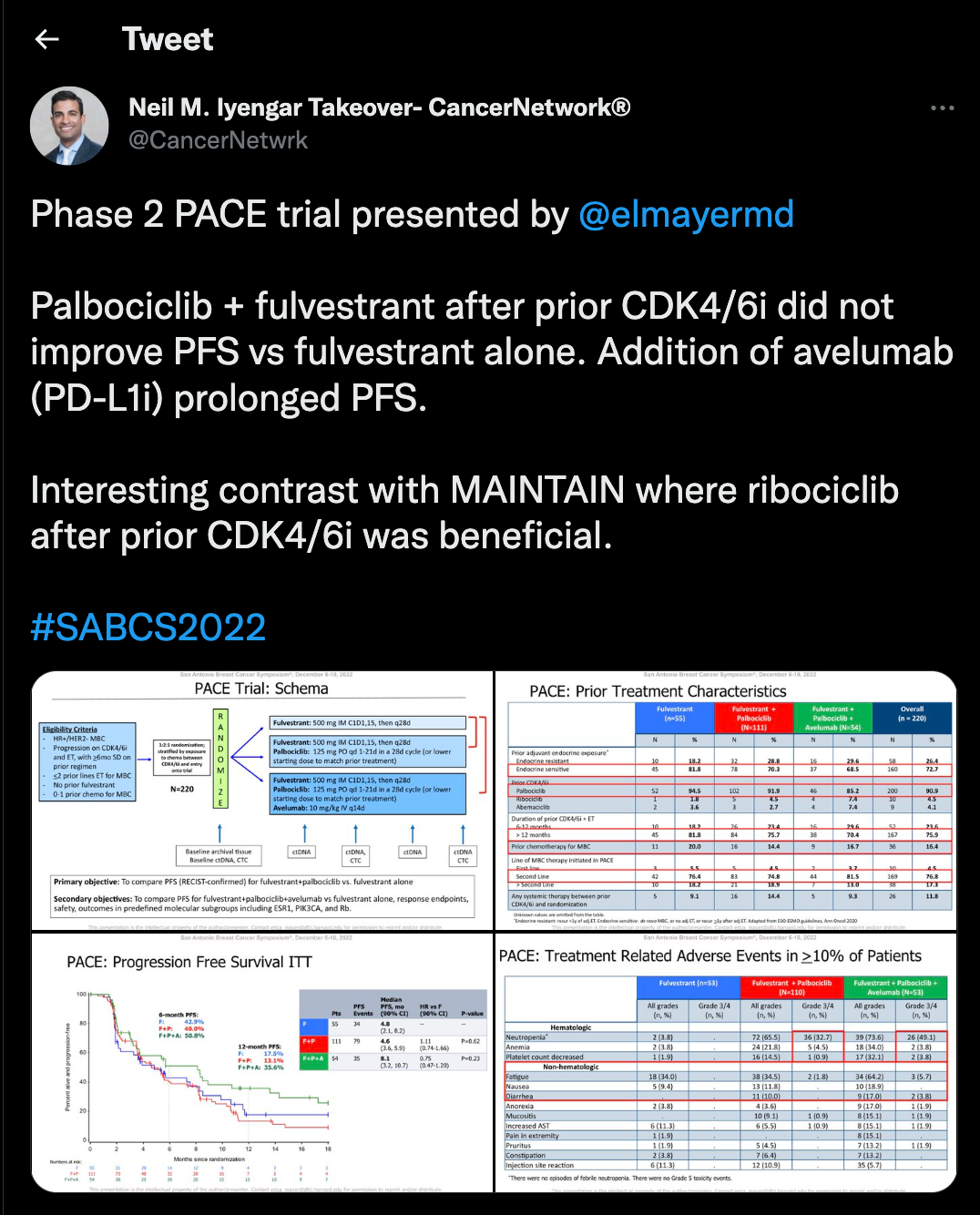
The median PFS in the fulvestrant/palbociclib arm was 4.6 months (90% CI, 3.6-5.9; HR, 1.11; 90% CI, 0.74-1.66; P = .62) vs 4.8 months (90% CI, 2.1-8.2) in the fulvestrant alone arm and 8.1 months (90% CI, 3.2-10.7) in the triplet regimen arm (HR, 0.75; 90% CI, 0.47-1.20; P = .23).
The ORR in the fulvestrant group was 10.8%, 13.7% for fulvestrant plus palbociclib, and 17.9% in the triplet combination arm. The clinical benefit rate was 29.1% in the fulvestrant arm, 32.4% in the fulvestrant plus palbociclib arm, and 35.2% in the triplet combination arm.
The median OS was 27.5 months (90% CI, 21.1-38.0) in the fulvestrant arm, 24.6 months (90% CI, 21.5-33.3) in the fulvestrant plus palbociclib arm, and 42.5 months (90% CI, 26.8-46.0) in the triplet arm.
To view the full Twitter Takeover, search #CNRealtimeReport on Twitter
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.