Accelerated FDA Approval for CML Drug Ponatinib Sought
Ariad Pharmaceuticals has asked the FDA for an accelerated approval of the BCR-ABL inhibitor ponatinib, based on promising data from ongoing phase II trials.
Ariad Pharmaceuticals has asked the US Food and Drug Administration (FDA) for an accelerated approval of the BCR-ABL inhibitor ponatinib, based on promising data from ongoing phase II trials. Ponatinib is intended to treat patients with chronic myeloid leukemia (CML) who are resistant or intolerant to other tyrosine kinase inhibitors, and patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL).
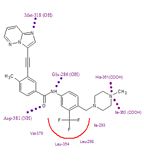
Ponatinib in it's binding site; amino acids with major interactions displayed, dotted lines represent hydrogen bonds
According to a press release from Ariad, the FDA will begin an immediate review of pontatinib; the company expects approval and commercial launch of the drug in the first few months of 2013. Ariad says that “ponatinib targets not only native BCR-ABL but also its isoforms that carry mutations that confer resistance to treatment with existing tyrosine kinase inhibitors, including the T315I mutation for which no effective therapy currently exists.”
In the ongoing phase II PACE trial, 70% of patients with that T315I mutation achieved a major cytogenetic response (MCyR) at an interim analysis. Fifty-four percent of all chronic-phase CML patients resistant or intolerant to other treatments achieved an MCyR. Jorge Cortes, MD, of the University of Texas MD Anderson Cancer Center in Houston, reported results on 444 patients in the trial at the American Society of Clinical Oncology annual meeting in June. He noted then that the responses appear to be durable, with 93% of CML patients still in MCyR after 1 year.
The patients in PACE were divided into six cohorts based on disease type; 267 of the 444 total were chronic-phase CML patients, of whom 144 achieved an MCyR, and 44% achieved a complete cytogenetic response over a median follow-up period of 10.1 months. Of the 64 evaluable chronic-phase CML patients with the T315I mutation, 66% achieved a complete cytogenetic response. Overall, 93% of the trial’s participants had previously received treatment with at least two tyrosine kinase inhibitors, and 58% had received at least three such drugs.
Safety of ponatinib was also found to be acceptable in the PACE trial, with thrombocytopenia, rash, dry skin, and abdominal pain representing the most common adverse events. Pancreatitis, which was found to be the dose-limiting toxicity of the drug in phase I trials, occurred in 6% of patients across all cohorts. A phase III trial comparing ponatinib to imatinib is now recruiting patients.
As Cancer Networkreported in June, Olatoyosi Odenike, MD, of the University of Chicago, said that ponatinib could be a good addition to the arsenal of drugs to fight CML. “This provides a new treatment option for the heavily pretreated patients with CML, including patients with the T315I mutation,” he said.
A collaborator company of Ariad’s, MolecularMD Corp, also submitted a premarket approval application to the FDA for a diagnostic test to be used in concert with ponatinib; the test would determine which CML and ALL patients have the T315I mutation, making them candidates for the drug. Ariad will also submit an application to the European Medicines Agency before the end of this quarter.