Although both breast-conserving surgery and mastectomy generally provide excellent local-regional control of breast cancer, local-regional recurrence (LRR) does occur. Predictors for LRR include patient, tumor, and treatment-related factors. Salvage after LRR includes coordination of available modalities, including surgery, radiation, chemotherapy, and hormonal therapy, depending on the clinical scenario. Management recommendations for breast cancer LRR, including patient scenarios, are reviewed, and represent evidence-based data and expert opinion of the American College of Radiology Appropriateness Criteria Expert Panel on LRR. The American College of Radiology Appropriateness Criteria are evidence-based guidelines for specific clinical conditions that are reviewed every 2 years by a multidisciplinary expert panel. The guideline development and review include an extensive analysis of current medical literature from peer reviewed journals and the application of a well-established consensus methodology (modified Delphi) to rate the appropriateness of imaging and treatment procedures by the panel. In instances in which evidence is lacking or not definitive, expert opinion may be used to recommend imaging or treatment.
Summary of Literature Review
Introduction
For the greater part of recent decades, the major focus of radiation oncology in the management of breast cancer has been on performing both randomized and nonrandomized trials comparing breast-conserving therapy (BCT) using surgery and radiation with the more traditional modified radical mastectomy (MRM). With data confirming equivalent survival with these two local-regional therapies in the management of early-stage breast cancer, attention was then refocused on identifying factors (pathologic, patient, or therapy-oriented) that predicted the success or failure of the local-regional treatment.
Twenty-year rates of local-regional recurrence (LRR) in the intact breast after BCT have been reported to be 8.8%.[1] Similarly low long-term crude recurrence rates (13%) for BCT were reported in the Danish trial as compared with mastectomy (21%).[2] The relationship of local recurrence to the development of distant failure and death from disease is being well studied. In early studies, the survival rate with salvage surgery for failures after BCT was 50% or higher at 5 years. These local recurrences were thought less likely to result in subsequent distant disease from local-regional failures when following BCT than when following mastectomy; after the latter, they were readily linked with the development of distant disease and death.[3]
Today, any discussion about treatment for LRR must be considered in the context of advances in systemic chemotherapy, hormonal therapy, and targeted agents such as trastuzumab in the management of breast cancer, along with changes in patterns of survival and disease-free survival. Women who decades ago would have died rapidly from distant disease may now exhibit a local-regional failure as a result of prolonged survival. Outcomes of these LRR failures without distant disease have been re-examined. Analysis of two prospective randomized trials from the European Organisation for Research and Treatment of Cancer (EORTC) and the Danish Breast Cancer Group, which compared mastectomy vs BCT in patients with similar disease stages managed using similar systemic therapy showed almost identical 5-year actuarial local-regional control and survival rates following salvage procedures for early local-only failures, in both the BCT and mastectomy study arms.[4] Thus, the relationship between local failure and distant failure must be reanalyzed in both patients treated with mastectomy and those treated with BCT.
As the radiation oncology field of breast cancer therapy continues to advance, with examination of alternate fractionation schema and methods such as hypofractionation and accelerated partial breast irradiation (APBI), the importance of obtaining local control with initial treatment must remain important-not only to prevent either loss of the breast in the BCT patients or painful and difficult-to-control local failure in the mastectomy patients, but also to potentially decrease subsequent distant metastases that may be associated with these local failures. Data from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) demonstrate that treatments resulting in improved local control may lead to a decrease in breast cancer mortality.[5] The overview analysis also suggests that avoidance of local recurrence after breast-conserving surgery (BCS) and radiation, and avoidance of local recurrence elsewhere after mastectomy, such as in the chest wall or regional nodes, are of comparable relevance to 15-year breast cancer mortality. As systemic therapeutic regimens become more effective at reducing the risk of distant disease, the goal of ensuring local-regional control takes on a potentially greater importance.
Randomized trial updates demonstrated the value of postmastectomy radiation therapy (PMRT) in patients with stage II disease with one to three positive lymph nodes. The Danish Cooperative Breast Cancer Group 82b and 82c trials and the Vancouver British Columbia trial evaluated local control and survival in mastectomy patients with one to three positive lymph nodes treated with systemic chemotherapy with or without PMRT.[6,7] Radiation led to both decreased LRR and improved survival. Additional analysis of patients in the Danish trial who underwent a more complete axillary dissection of eight or more removed lymph nodes also revealed both a significantly lower 15-year risk of LRR and improvement in survival in patients receiving radiation.[8] Similarly, the value of regional nodal irradiation was demonstrated in the results from the MA 20 study in patients undergoing BCT.[9]
Recurrence After Breast-Conserving Surgery and Radiation
Predictors for local-regional failure after both BCS and radiation can be divided into three broad categories: patient, tumor, and therapeutic factor. Young age at the time of diagnosis (patients in their 30s and 40s) appears to be a strong predictor.[10-13]
Several studies have found that positive microscopic margins, gross multifocality, and an extensive intraductal component are associated with a higher risk of recurrence in the conserved breast. Additionally, larger tumor size and lymphatic vessel invasion have been reported as risk factors for ipsilateral breast tumor recurrence (IBTR).[10,13-15] Newer studies suggest that the molecular subtype may also impact local recurrence, with both triple-negative (estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor type 2 [HER2/neu]) and HER2-enriched (HER2+) subtypes associated with higher rates of local and regional relapse.[16,17]
The third category of risk factors for IBTR consists of therapeutic factors, most importantly the omission of breast radiation. Numerous studies have demonstrated that radiation therapy (RT) significantly reduces the risk of cancer recurrence in the breast.[18-20] Patients who receive systemic chemotherapy or hormone therapy appear to have higher local control rates, all else being equal, than those who do not.[14,19,21] The addition of a radiation boost to the lumpectomy cavity may decrease the incidence of a recurrence in the conserved breast, particularly in women younger than 40 years of age.[11]
An increasing number of women are treated with APBI with either interstitial or balloon brachytherapy or external beam treatment. A limited long-term follow-up study regarding pattern of failure in breast or nodal regions is generally lacking. However, existing data do suggest that the rate of IBTR is comparable to the rate for whole-breast irradiation (WBI), with 10-year recurrence rates reported to be up to 6%.[17,22-25] An update of the American Society of Breast Surgeons MammoSite brachytherapy trial evaluated patients treated with balloon brachytherapy APBI. With a median of 53.5 months of follow-up, the 5-year actuarial IBTR rates were 2.59%, 5.43%, and 5.28%, respectively, for patients in the “suitable,” “cautionary,” and “unsuitable” categories, as defined by the American Society for Radiation Oncology (ASTRO) consensus panel on APBI (P = .1884). The only factor on univariate analysis associated with development of an IBTR was receptor-negative disease.[26] A matched-pair analysis of 199 patients who received interstitial APBI vs WBI showed no differences in 12-year rates of local recurrences (3.8% vs 5%), regional recurrences (0% vs 1.1%), or cause-specific survival (78% vs 71%).[27]
Neoadjuvant therapy is increasingly used in breast cancer patients in whom it is known that systemic therapy will be needed in an adjuvant setting or to increase chances of success with BCS. Data suggest that BCT can be used after neoadjuvant therapy with acceptable local control.[28,29] In the NeOAdjuvant Herceptin (NOAH) trial, 235 patients with HER2-positive locally advanced or inflammatory breast cancer were randomized to treatment with neoadjuvant trastuzumab plus chemotherapy or chemotherapy alone. Only 6 of 235 patients (2.5%) experienced either an IBTR or chest wall relapse with a median follow-up time of 3.2 years, with the rate being similar in both BCT and mastectomy groups. None of the four patients undergoing BCT after chemotherapy with trastuzumab experienced an IBTR.[30]
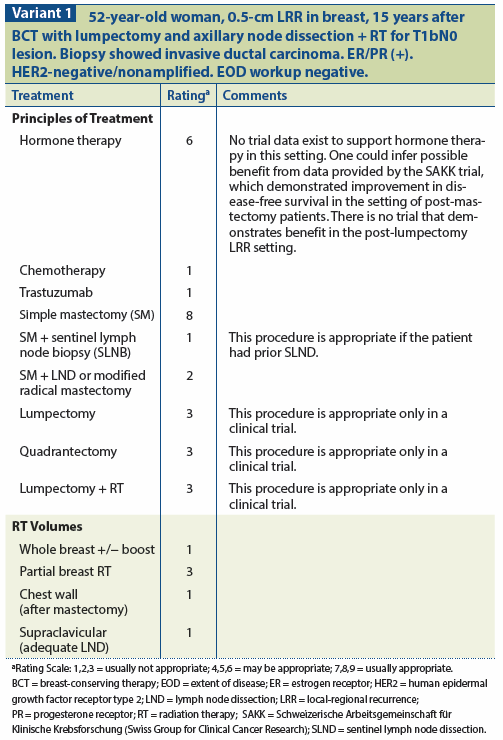
The 5-year survival rate following an IBTR in patients undergoing BCT is 76.6% for node-negative patients and 59.9% for node-positive patients.[31,32] The generally recommended treatment for locally recurrent breast cancer after BCT using WBI is salvage mastectomy, although a repeat attempt at breast conservation may also be possible in select cases. The phase II trial 1014, which is ongoing, is evaluating repeat BCS followed by 3D conformal partial breast re-irradiation. (See Variant 1.)
Limited data exist on salvage of patients who develop IBTR after APBI. Repeat BCS or mastectomy has been shown to produce excellent salvage rates comparable to the low rate of failures after WBI, but patient numbers are small.[23,24] Out of 1,440 patients treated with balloon brachytherapy APBI, the rate of IBTR at 5 years was shown to be 3.6% for invasive breast cancer and 3.3 % for ductal carcinoma in situ (DCIS). The 3-year disease-free survival and overall survival rates were 58% and 80%, respectively, after salvage mastectomy or repeat breast conservation. In this trial, 74% of the patients underwent salvage mastectomy and 26% received BCT.[33]
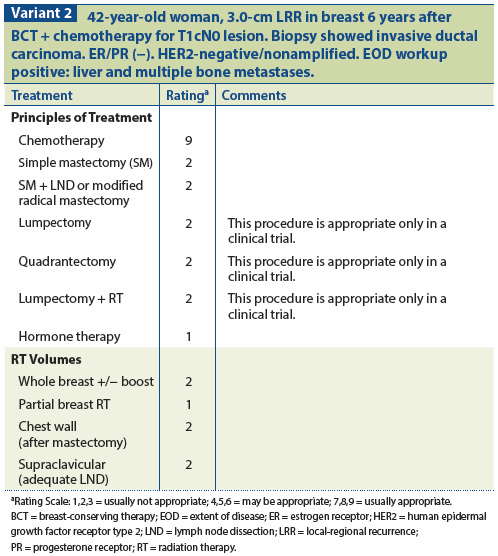
The incidence of any nodal recurrence in breast-conserving series is low.[34] However, involvement of the nodes has a ssignificant impact on outcome; therefore, assessment of the axillary status for an invasive local recurrence of the breast should be considered. The role of sentinel lymph node biopsy (SLNB) in this scenario remains to be defined. Preliminary data indicate that SLNB may be performed at the time of salvage surgery, given that previous breast conservation or axillary surgery may not be a contraindication to SLNB.[35] Because of the relationship between local recurrence and distant failure, systemic therapy must also be considered in the treatment program for this patient group. (See Variant 2.)
To date, no published series has shown a statistically significant improvement in subsequent outcome with the administration of salvage chemotherapy or hormonal intervention at the time of local recurrence. Factors to be considered in this clinical decision include: (1) prior systemic therapy, if any; (2) extent of recurrence; (3) time from initial treatment to recurrence; (4) tumor hormone receptor status; (5) patient age; (6) tumor size; (7) margin status; and (8) general medical condition.[31,32]
The National Surgical Adjuvant Breast and Bowel Project (NSABP) is evaluating the benefit of adjuvant chemotherapy following radical resection of recurrent LRR breast cancer. This prospective randomized trial is currently open to accrual.[36]
Some patients who present with an IBTR following BCT may have a new primary tumor as opposed to a true local recurrence.[37,38] The IBTR tumor is defined as a new primary if it is distinctly different from the original tumor with respect to histology subtype, if it presents in a different location in the breast, or if it is of different clonality. The time between the original primary and the second tumor is generally considerably greater for new primaries compared with true recurrences (average, 55 months vs 33 months).[37] In patients receiving RT as part of BCT, 10-year overall survival rates (75% vs 55%) and distant disease-free survival rates (85% vs 41%) tend to be much better for patients with new primaries than for those with true recurrences.[38] Thus, the diagnosis of a new primary as opposed to a true recurrence implies a different natural history and prognosis and has different implications for therapeutic management. Unfortunately, most series addressing breast tumor recurrences do not adequately distinguish between the two entities. This may be of particular importance to breast cancer management in young women with BRCA1/BRCA2 gene mutations, who are at increased risk for breast tumor recurrences due to new primaries.[39]
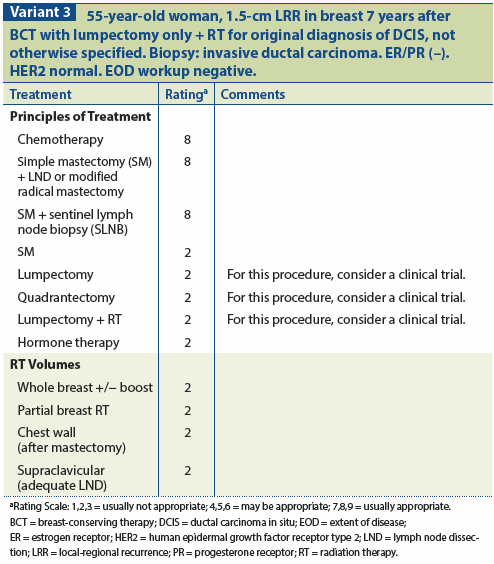
Patients with DCIS who undergo BCT and subsequently sustain a recurrence in the treated breast appear to have an excellent outcome following salvage therapy.[40] In most series, about half of the recurrences are invasive, with the other half recurring as DCIS. Nevertheless, almost all of these patients can be cured by mastectomy.
Outcomes of IBTR after breast conservation were analyzed in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17 and B-24 trials. The results of B-17 demonstrated that the 15-year cumulative incidence of invasive IBTR in patients treated with BCS was 19% with lumpectomy alone and 8.9% with lumpectomy and RT. In B-24, the 15-year cumulative incidence of invasive IBTR in patients treated with lumpectomy alone was 10% in lumpectomy and RT vs 8.5% with lumpectomy and tamoxifen. In B-17, for patients with noninvasive DCIS, the incidence of IBTR was 15.7% for those who underwent lumpectomy only and 8.8% for those treated with lumpectomy and RT. In B-24, the incidence of IBTR was 8.3% for patients managed with lumpectomy and RT and 7.5% for those treated with lumpectomy and tamoxifen. The probability of cancer-related death was 10.4% at 10 years after an invasive recurrence vs 2.7% after a noninvasive (DCIS) IBTR.[40] (See Variant 3.)
Recurrence After Mastectomy
Risk factors for local-regional failure following mastectomy can also be divided into clinical, pathological, and treatment-related categories. Young age (< 35 years), nodal status, hormone receptor status, tumor size, lymphovascular invasion, multicentricity, and adequacy of nodal dissection as measured by the number of removed lymph nodes are all risk factors for postmastectomy recurrence.[41-43] Elective PMRT reduces this risk.[44] In the subgroup of patients with one to three positive nodes, there is controversy regarding both the risk of chest wall recurrence and the need for PMRT, due to an unclear impact on survival and the potential for increased toxicity despite increased local control. (See the Appropriateness Criteria® topic, “Postmastectomy Radiotherapy.”)
Systemic therapy appears to have an impact on local-regional control. In the most recent meta-analysis of systemic therapy from the EBCTCG, 5 years of tamoxifen therapy reduced the local recurrence rate by about one-half in women with hormone receptor–positive disease (local recurrence ratio of 0.47), whereas, irrespective of hormone receptor status, polychemotherapy reduced it by about one-third (local recurrence ratios, 0.63–0.70, depending on patient age).[45]
Five-year survival rates after LRR range from 35% to 75%, and 10-year survival rates range from 25% to 55%. Long-term control of local-regional disease is achieved in only 45% to 70% of patients. Most patients with early LRR develop distant metastases,[46] but a favorable subgroup exists with a lower risk of distant metastases and improved 5- and 10-year survival rates.
Prognostic factors include the extent of disease (EOD) initially and at recurrence; the disease-free interval; tumor grade; ER status; and the use of surgical excision, radiation, and hormonal therapy. Patients with uncontrolled local-regional disease are usually symptomatic, are more likely to develop distant metastases, and die sooner than patients who have controlled LRR. Consequently, aggressive attempts at controlling the LRR are warranted.
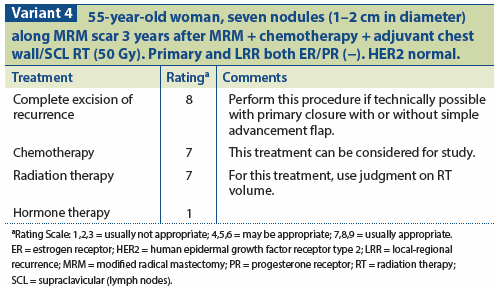
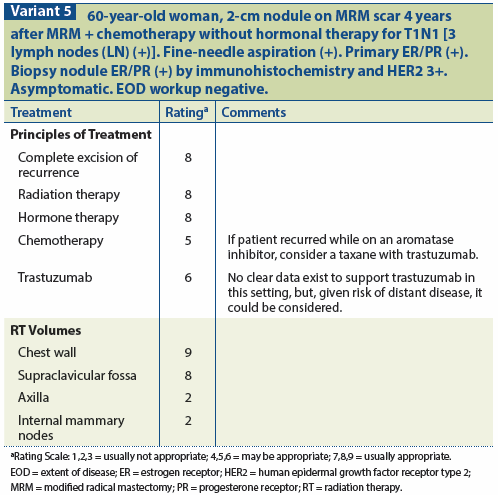
A multidisciplinary approach is required for the management of a chest wall recurrence after mastectomy. (See Variant 4 and Variant 5.)
Surgical resection should be performed if the size and location of the recurrence permit it. LRRs are managed with irradiation in patients who have not received prior RT.[41] LRR after mastectomy is a harbinger of distant metastases, therefore systemic treatment should also be considered. If the patient is ER-positive, then tamoxifen, an aromatase inhibitor (depending on the patient’s menopausal status), or ovarian ablation may be used. If the patient is ER-negative, then chemotherapy may be administered.[36] Although it is a reasonable treatment option, historically chemotherapy has not been proven to have an impact on patients’ overall survival after a recurrence, and it has not been well studied. However, data from the Chemotherapy as Adjuvant for LOcally Recurrent Breast Cancer (CALOR) trial that were presented in 2012 at the San Antonio Breast Cancer Symposium show a benefit to chemotherapy, predominantly in ER-negative women.
Treatment Guidelines After Breast-Conserving Therapy
For patients who fail to respond to BCT that includes standard WBI and an axillary node dissection, simple mastectomy is recommended as the local treatment of choice when the failure is confined to the breast parenchyma and is operable.[47] The role of partial breast irradiation in this setting is currently under investigation, and consideration should be given to enrolling the patient in an available clinical trial. The Radiation Therapy Oncology Group (RTOG) phase II trial 1014 is currently open for accrual. It uses 3D conformal partial breast irradiation for patients with an in-breast local recurrence ≤ 3 cm and three or more positive lymph nodes without extranodal extension.
In the clinical situation involving recurrence in the treated breast as well as a supraclavicular nodal failure, radiation to the untreated supraclavicular area plus chemotherapy is the recommendation. Although this pattern of recurrence is not common, it is viewed as systemic failure based on existing evidence. Similarly, for patients with clear distant metastases as well as local failure, primary systemic management is recommended rather than mastectomy.
Summary
- Five-year actuarial LRR and survival rates following salvage procedures for early local-only failures are similar in patients undergoing mastectomy vs BCT.
- Salvage mastectomy is generally recommended for locally recurrent breast cancer after BCS. Repeated attempts at breast conservation may be considered, preferably through participation in a clinical trial.
- To date, no published series has shown statistically significant improvement in outcome with salvage chemotherapy or hormonal therapy in the case of local recurrence after BCT.
- Multidisciplinary management of chest wall recurrence after mastectomy is warranted, including surgery, RT, and systemic therapy. In the absence of distant metastasis, aggressive attempts at salvage should be pursued.
- Multidisciplinary management of isolated axillary or supraclavicular nodal recurrence is warranted where feasible with surgery and RT, although risk of systemic failure is high. The benefit of systemic therapy in this setting remains to be determined.
In the rare clinical situation of local recurrence in a patient whose initial treatment consisted only of a wide local excision without radiation or axillary dissection, treatment options include simple excision or lumpectomy, axillary nodal evaluation, and RT in the absence of distant metastasis.
Given the situation of a patient with recurrent DCIS treated initially with lumpectomy plus RT only, simple excision is recommended.
Treatment Guidelines for Local Recurrence After Mastectomy
Treatment options for LRR following mastectomy include surgery, RT, chemotherapy, hormonal therapy, or a combination of modalities. Patients experiencing LRR after mastectomy should undergo a workup for metastatic disease. In the absence of distant metastases, it is important to aggressively attempt salvage. When possible, surgical excision followed by RT to the involved chest wall and regional lymphatics is the standard treatment.
Haffty et al[48] reported overall survival rates of 46% at 5 years and 28% at 10 years after chest wall recurrence for patients treated with full-course external beam irradiation. Ten-year local-regional disease control was achieved in 79% of patients, with a distant metastasis–free survival rate of 49% at 5 years and 40% at 10 years. In this series, HER2 status was the only significant factor affecting local-regional progression. Patients with HER2-positive disease had a local-regional progression-free rate of 59%, compared with 92% for patients with HER2-negative disease. Both PR-positive status and time longer than 2 years from the original diagnosis to chest wall recurrence were associated with favorable distant metastasis–free and long-term survival. Along with disease-free interval, the adequacy of local control for LRR has also been shown to have a favorable impact on long-term survival.
Isolated Axillary and Supraclavicular Nodal Failures
Isolated nodal recurrences in the axillary or supraclavicular nodal regions occur less frequently than chest wall or in-breast recurrences. In a review by Walsh et al of 1,614 breast cancer patients undergoing either lumpectomy or mastectomy, only 14 patients (0.9%) developed an ipsilateral nodal recurrence after axillary dissection.[49] Isolated supraclavicular recurrence is similarly uncommon.
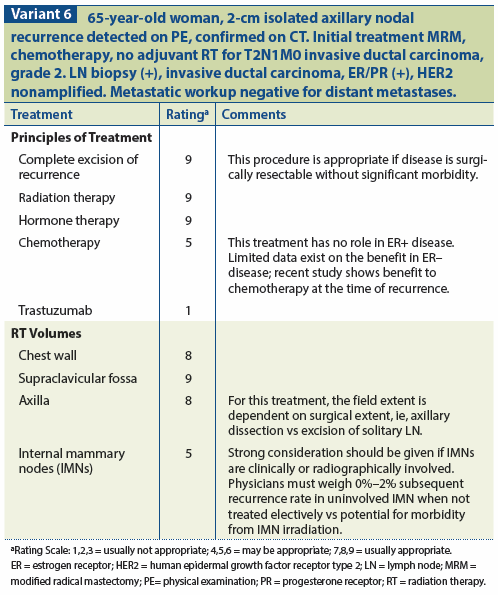
If feasible, surgery is usually used as the initial treatment modality. Radiation therapy is generally used after surgery if the patient has not received prior RT. Systemic therapy may also be incorporated in the salvage therapy, although the role of systemic therapy in this setting is unclear. Surgery, however, may not be technically feasible due to prior axillary dissection. Likewise, supraclavicular disease may not be amenable to surgical resection due to potential postoperative morbidity. In addition, patients who have received prior axillary or supraclavicular RT usually are not candidates for re-irradiation. Both isolated axillary and supraclavicular relapses are generally associated with a poor long-term survival due to high rate of subsequent distant metastasis.[49,50] However, subsets of patients with favorable features have been identified, including those with only a single axillary nodal recurrence, disease-free intervals greater than 1 year, and attainment of local control, with 10-year survival reported at 69%.[51] In the absence of distant metastasis, vigorous attempts at salvage should be pursued. (See Variant 6.)
The American College of Radiology seeks and encourages collaboration with other organizations on the development of the ACR Appropriateness Criteria through society representation on expert panels. Participation by representatives from collaborating societies on the expert panel does not necessarily imply individual or society endorsement of the final document.
Financial Disclosure: Deborah Toppmeyer, MD, discloses that her husband is an employee of Novartis who has stock ownership exceeding $10,000. The remaining authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Copyright © 2014 American College of Radiology. Reprinted with permission of the American College of Radiology.
For additional information on ACR Approp-riateness Criteria®, refer to www.acr.org/ac.
References:
1. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-32.
2. Blichert-Toft M, Nielsen M, During M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47:672-81.
3. Moran MS, Haffty BG. Local-regional breast cancer recurrence: prognostic groups based on patterns of failure. Breast J. 2002;8:81-7.
4. van Tienhoven G, Voogd AC, Peterse JL, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer. 1999;35:32-8.
5. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-106.
6. Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer-risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147-55.
7. Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116-26.
8. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247-53.
9. Whelan TJ, Olivotto I, Ackerman I, et al. NCIC-CTG MA.20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29(suppl):Abstr LBA1003.
10. Arriagada R, Le MG, Contesso G, et al. Predictive factors for local recurrence in 2006 patients with surgically resected small breast cancer. Ann Oncol. 2002;13:1404-13.
11. Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259-65.
12. de Bock GH, van der Hage JA, Putter H, et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer. 2006;42:351-6.
13. Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688-97.
14. Freedman GM, Hanlon AL, Fowble BL, et al. Recursive partitioning identifies patients at high and low risk for ipsilateral tumor recurrence after breast-conserving surgery and radiation. J Clin Oncol. 2002;20:4015-21.
15. Kreike B, Hart AA, van de Velde T, et al. Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;71:1014-21.
16. Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684-91.
17. Solin LJ, Hwang WT, Vapiwala N. Outcome after breast conservation treatment with radiation for women with triple-negative early-stage invasive breast carcinoma. Clin Breast Cancer. 2009;9:96-100.
18. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-41.
19. Potter R, Gnant M, Kwasny W, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys. 2007;68:334-40.
20. Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997-1003.
21. Livi L, Paiar F, Saieva C, et al. Survival and breast relapse in 3834 patients with T1-T2 breast cancer after conserving surgery and adjuvant treatment. Radiother Oncol. 2007;82:287-93.
22. Antonucci JV, Wallace M, Goldstein NS, et al. Differences in patterns of failure in patients treated with accelerated partial breast irradiation versus whole-breast irradiation: a matched-pair analysis with 10-year follow-up. Int J Radiat Oncol Biol Phys. 2009;74:447-52.
23. Arthur DW, Winter K, Kuske RR, et al. A phase II trial of brachytherapy alone after lumpectomy for select breast cancer: tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2008;72:467-73.
24. Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma-5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694-702.
25. Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/ II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120-7.
26. Shaitelman SF, Vicini FA, Beitsch P, et al. Five-year outcome of patients classified using the American Society for Radiation Oncology consensus statement guidelines for the application of accelerated partial breast irradiation: an analysis of patients treated on the American Society of Breast Surgeons MammoSite Registry Trial. Cancer. 2010;116:4677-85.
27. Shah C, Antonucci JV, Wilkinson JB, et al. Twelve-year clinical outcomes and patterns of failure with accelerated partial breast irradiation versus whole-breast irradiation: results of a matched-pair analysis. Radiother Oncol. 2011;100:210-4.
28. Peintinger F, Symmans WF, Gonzalez-Angulo AM, et al. The safety of breast-conserving surgery in patients who achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2006;107:1248-54.
29. Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96-102.
30. Semiglazov V, Eiermann W, Zambetti M, et al. Surgery following neoadjuvant therapy in patients with HER2-positive locally advanced or inflammatory breast cancer participating in the NeOAdjuvant Herceptin (NOAH) study. Eur J Surg Oncol. 2011;37:856-63.
31. Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466-73.
32. Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028-37.
33. Shah C, Vicini F, Keisch M, et al. Outcome after ipsilateral breast tumor recurrence in patients who receive accelerated partial breast irradiation. Cancer. 2012;118:4126-31.
34. Harris EE, Hwang WT, Seyednejad F, Solin LJ. Prognosis after regional lymph node recurrence in patients with stage I-II breast carcinoma treated with breast conservation therapy. Cancer. 2003;98:2144-51.
35. Port ER, Garcia-Etienne CA, Park J, et al. Reoperative sentinel lymph node biopsy: a new frontier in the management of ipsilateral breast tumor recurrence. Ann Surg Oncol. 2007;14:2209-14.
36. Wapnir IL, Aebi S, Gelber S, et al. Progress on BIG 1-02/IBCSG 27-02/NSABP B-37, a prospective randomized trial evaluating chemotherapy after local therapy for isolated locoregional recurrences of breast cancer. Ann Surg Oncol. 2008;15:3227-31.
37. Nishimura S, Takahashi K, Akiyama F, et al. Classification of ipsilateral breast tumor recurrence after breast-conserving therapy: new primary cancer allows a good prognosis. Breast Cancer. 2005;12:112-7.
38. Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281-9.
39. Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:5887-92.
40. Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478-88.
41. Buchanan CL, Dorn PL, Fey J, et al. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg. 2006;203:469-74.
42. Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247-54.
43. Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852-8.
44. Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419-26.
45. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-717.
46. Schmoor C, Sauerbrei W, Bastert G, Schumacher M. Role of isolated locoregional recurrence of breast cancer: results of four prospective studies. J Clin Oncol. 2000;18:1696-708.
47. Carlson RW, Anderson BO, Burstein HJ, et al. Breast cancer. J Natl Compr Canc Netw. 2005;3:238-89.
48. Haffty BG, Hauser A, Choi DH, et al. Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer. 2004;100:252-63.
49. Walsh N, Kiluk JV, Sun W, et al. Ipsilateral nodal recurrence after axillary dissection for breast cancer. J Surg Res. 2012;177:81-6.
50. Kiricuta IC, Willner J, Kolbl O, Bohndorf W. The prognostic significance of the supraclavicular lymph node metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 1994;28:387-93.
51. Willner J, Kiricuta IC, Kolbl O. Locoregional recurrence of breast cancer following mastectomy: always a fatal event? Results of univariate and multivariate analysis. Int J Radiat Oncol Biol Phys. 1997;37:853-63.