The purpose of this article is to present an updated set of American College of Radiology consensus guidelines formed from an expert panel on the appropriate use of radiation therapy in postprostatectomy prostate cancer. The American College of Radiology Appropriateness Criteria are evidence-based guidelines for specific clinical conditions that are reviewed every 3 years by a multidisciplinary expert panel. The guideline development and review include an extensive analysis of current medical literature from peer-reviewed journals and the application of a well-established consensus methodology (modified Delphi) to rate the appropriateness of imaging and treatment procedures by the panel. In those instances where evidence is lacking or not definitive, expert opinion may be used to recommend imaging or treatment. Recent and relevant literature reviewed by the panel led to establishment of criteria for appropriate use of radiation therapy in postprostatectomy prostate cancer. The discussion includes treatment technique, appropriate dose, field design, and the role of prostate-specific antigen (PSA). Ratings and commentary of the panel on multiple treatment parameters were used to reach consensus. Patients with high-risk pathologic features benefit from postprostatectomy radiation therapy.
Summary of Literature Review
Introduction/Background
Radical prostatectomy (RP) and radiation therapy (RT), including brachytherapy, are the primary treatment options for organ-confined prostate cancer (T1-2, stages I or II). Eventually, 50% to 70% of postprostatectomy patients with high-risk pathologic features, such as a positive margin, extracapsular extension (ECE), and/or seminal vesicle involvement (SVI), will develop biochemical failure (BF).[1] Thus, RT may play a role either immediately following prostatectomy, based on various known high-risk pathologic features, or at the time of BF.[2-5]
There are three main clinical scenarios in which RT is given after RP: (1) adjuvant radiotherapy (ART) for men with undetectable or barely detectable levels of prostate-specific antigen (PSA; < 0.2 ng/mL) who have high-risk pathologic features; (2) salvage radiotherapy (SRT) for men with undetectable or barely detectable PSA levels (< 0.2 ng/mL) immediately postoperatively but whose PSA rises at some later date-a delayed rise in PSA (DR-PSA); and (3) SRT for men whose PSA remains at 0.2 ng/mL or above postoperatively-a persistently detectable PSA (PD-PSA).
The purpose of distinguishing between ART and SRT is rooted in the observation that there are significant differences between the two groups in terms of prognosis after RT, dose of RT administered, and prognostic factors. The further subdivision of salvage patients into two groups, those with a DR-PSA vs those with a PD-PSA, is useful because their outcomes after RT appear to be different,[6-10] with a worse prognosis for those with a PD-PSA. In general, the earlier the rise in PSA after RP, the worse the outcome because of a higher risk of metastatic disease.
Adjuvant Radiotherapy
The rationale for administering ART after RP is predicated on the assumption that microscopic local disease remains. Local therapy reduces the rate of recurrence in the prostate bed and may reduce the risk that the residual nidus of prostate cancer will disseminate distantly. The decision to administer ART is based on the presence of high-risk pathologic findings in the prostatectomy specimen. The primary high-risk features are ECE, positive margins (prostate cancer at the margin of resection), and SVI.[11] The rate of adverse pathologic findings may vary considerably due to patient selection and prognostic factors, as well as surgical technique and pathologic evaluation, but adverse findings occur at approximate rates of 40% for ECE, 25% for margin positivity, 10% for SVI, and 5% for lymph node involvement (LNI).[12-22]
The prevalence of persistent local disease following RP is significant. Residual disease has been documented in approximately 50% of prostatectomy cases at autopsy,[23] and in biopsy specimens of the prostatic fossa and urethrovesical anastomosis.[11,24-26] Long-term follow-up has revealed that the risk of BF following prostatectomy is substantial. Various surgical series have reported that this risk continues to be present between 5 and 10 years after prostatectomy, with an average relative risk of 2%–3% per year, without reaching a plateau.[22,27-29] Late BFs are not insignificant and may eventually lead to the development of painful bony metastases in 50% of patients within 7–8 years.[30-32] ART has the potential to reduce failure and ultimately improve quality of life, leading to fewer local and systemic failures.[33] Failure may lead to the need for additional therapy using androgen deprivation, and the associated treatment side effects. Patients with a life expectancy of > 10 years should benefit from ART.
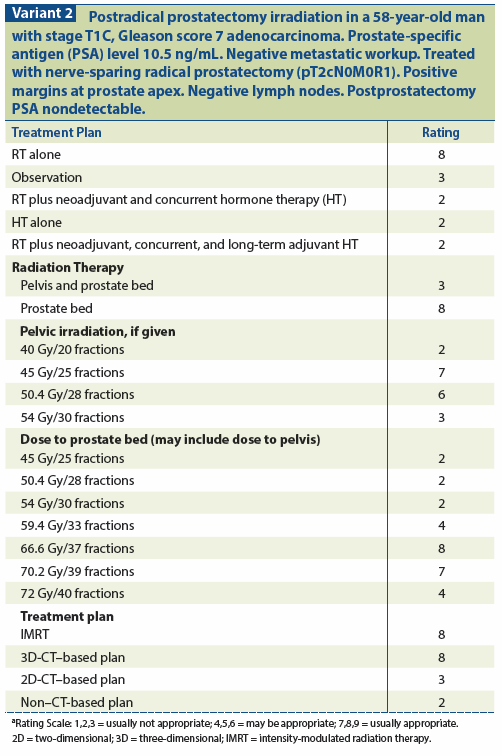
A powerful predictor of biochemical and local failure after prostatectomy is margin positivity. It is estimated that approximately 40% of men with a positive surgical margin will experience a rise in PSA to detectable levels within 5–10 years.[13,34-40] Other pathologic features that predict for BF include ECE, also referred to as extraprostatic extension, Gleason score ≥ 7, and SVI.[4,13,36,37,39-43] The extent of margin positivity is another factor shown to influence BF[37,44,45] that has only been examined in retrospective series. ART may have less of an effect in the case of a small focal positive margin in the absence of other unfavorable pathologic features.[46] In this setting, other factors, such as the degree of extraprostatic extension[47] and/or Gleason score ≥ 7, appear to contribute to a greater risk of BF and provide a stronger rationale for ART. Similarly, a focal area of ECE alone is associated with a lower risk of biochemical progression, as compared with more extensive ECE, but the risk will be higher when the ECE is accompanied by Gleason score ≥ 7 disease.
In the setting of negative margins and a rising PSA, a complete biochemical response to SRT is still achieved in the majority of cases, suggesting that local disease persists in the prostatic fossa only. A rising PSA after a negative margin has been associated with a worse prognosis in some prostatectomy series[48,49]; however, it should be kept in mind that not every micron of tissue in the prostatectomy specimen is pathologically assessed. The RT response data suggest that tumor cells were left behind (a focal positive margin) but were not identified on pathologic evaluation. The risk of local disease persistence when there is obvious ECE in addition to a Gleason score ≥ 7,[47] even with negative margins, is significant enough that ART should be considered.
Adjuvant radiotherapy outcomes
Many retrospective studies have examined the role of ART.[50-55] Three prospective randomized trials comparing prostatectomy alone with prostatectomy plus ART have been reported.[22,55,56] All three trials have demonstrated an improvement in biochemical control of approximately 20% when ART is employed, with one trial demonstrating an improvement in both metastasis-free and overall survival. The European Organisation for Research and Treatment of Cancer (EORTC) 22911 study included 972 patients with pT2-3 prostate cancer with at least one high-risk feature (ECE, positive margins, or SVI). Freedom from biochemical failure (FFBF) at 5 years was 53% in the RP-alone group vs 74% in the RP plus RT (60 Gy) group (P < .0001)[56,57] and at 10 years was 41% vs 61%, respectively.
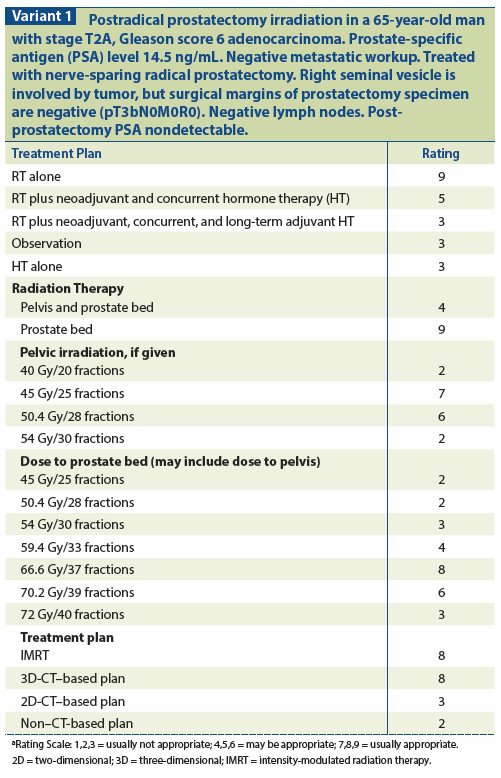
A similar study was conducted by the Southwest Oncology Group (SWOG).[33] A total of 473 patients with pathologically determined ECE, positive margins, and/or SVI were randomized to RT (at 60–64 Gy) vs observation. FFBF was significantly improved by the addition of radiation, from 38% to 61% at 5 years and from 23% to 47% at 10 years. This benefit was shared by each of the three pathologic risk groups. ART also obviated the need for androgen deprivation therapy (ADT) in some patients and delayed its use significantly (by 2.5 years) in others. The most recent update of this study demonstrates an improvement in its primary endpoint of metastasis-free survival, as well as in overall survival. With a median follow-up of 12.7 years, out of 425 evaluable patients, metastasis developed in 114 of 211 patients in the observation arm vs 93 of 214 patients who received early adjuvant therapy (P = .016). In addition, there have been 110 deaths in the observation arm vs 88 deaths among the irradiated patients (P = .023). Although ART initially resulted in some adverse impacts on quality of life, this difference disappeared by 2 years after treatment, and the irradiated patients actually fared better beyond 3 years after RT in terms of overall quality of life.[33] A third study (the German Cancer Society trial ARO 96-02) randomized 388 men with pT3 disease after prostatectomy and an undetectable postoperative PSA to either RT (60 Gy) or observation.[58] The 5-year FFBF rate was 54% in the RP-alone group vs 72% in the RP plus RT group (P = .0015). ART was very well tolerated, with a 0.3% rate of grade 3/4 late adverse events (see Variant 1 and Variant 2).
Salvage Radiotherapy
RT is given for salvage after RP in three settings: (1) for a DR-PSA after the PSA has dropped to an undetectable level immediately postprostatectomy, (2) for a PD-PSA after surgery, and (3) for treatment of a documented recurrence within the prostatic fossa. This distinction in categorizing patients suitable for SRT is relevant because the initial considerations in evaluation may be different. Furthermore, there are reported differences in outcome. However, many retrospective series were based on small patient numbers and did not separate these patients, making conclusions difficult.
Time to development of a rising PSA after prostatectomy, the prostatectomy Gleason score, and the PSA doubling time (PSADT) are independent predictors of distant metastasis and mortality.[30,31,59] When the time to BF is < 3 years (the PD-PSA patients would be included in this group), Gleason score is ≥ 8, and PSADT is < 9 months, the risk of death due to prostate cancer at 5 years is ≥ 19%. This risk increases to ≥ 74% at 10 years.[31] In another study, PSADT of < 6 months was associated with an increase in BF, distant metastasis, and prostate cancer–specific mortality.[59] PSADT has taken on much more importance over the last 5 years.[49,60,61] If the above parameters included a postoperative PSADT of < 3 months, nearly 50% would die within 5 years. PSA kinetics prior to prostatectomy may also be an independent determinant of mortality.[62-64] A rapidly rising PSA prior to RP or RT connotes a poor prognosis and is suggestive of occult metastatic disease even if the metastatic workup is negative. Nonetheless, salvage RT has the potential to improve prostate cancer–specific survival rates with short PSADT, as reported by Trock et al.[65] Thus, patients with short PSADT, although their prognosis is poorer than it would be otherwise, should be considered for SRT. Although the ability to predict progression after SRT has improved, definitive statements regarding optimal treatment regimens are difficult due to the absence of contemporary prospective clinical trials. There is a need to optimize treatment selection, with the goal of prolonging survival without unnecessary toxicity, particularly in the setting of rapid PSA kinetics and negative metastatic workup.
Factors indicating that postprostatectomy RT for a PD-PSA might be beneficial include extensive extraprostatic extension (particularly in patients with high-grade disease) or positive margins. Other indicators that there may be disease in the prostatic fossa are SVI, a cut-through of the prostate (a partial prostatectomy when there is evidence of prostate remaining, from palpation, biopsy, or imaging), or incomplete removal of the seminal vesicles in the setting of T3 disease (especially with ECE at the base or with SVI). In the absence of these features, and with a PSA that rises quickly (doubling time < 6 months), the probability of distant metastasis is high,[30,60,66-68] and SRT may be less beneficial.
The results of SRT have been relatively poor, with 5-year FFBF rates in most series ranging from 10% to 66%.[7-10,48,67,69-74] The following factors have been correlated with worse FFBF rates: Gleason score > 7, SVI, high pre-RT PSA level (> 1 ng/mL to > 2.5 ng/mL), short PSADT, negative prostatectomy margins, treatment for a PD-PSA (vs a DR-PSA), a palpable prostatic fossa mass, and RT dose < 65 Gy.
Salvage radiotherapy outcomes
In general, when the PSA remains detectable after RP, the risk of distant metastasis is greater than when the PSA becomes undetectable following prostatectomy and then rises later. Thus, outcomes of SRT in most series have been worse for patients with a PD-PSA compared with a DR-PSA.[7,8,10,71] However, some series have not found a significant difference in FFBF rates between the two groups.[9,49,73,75] Although distinguishing between the groups seems to be the most objective way of evaluating the utility of SRT, most of the studies reporting SRT outcomes do not separately analyze the DR-PSA and the PD-PSA patients. In addition, all of these studies are retrospective, and most include small numbers of patients.
As described above, the PSADT is an important predictor of SRT outcome. The shorter its duration, the greater the risk of death due to prostate cancer. A doubling time of ≤ 10 months in the setting of a DR-PSA or a PD-PSA indicates a higher likelihood of occult metastatic disease,[30,49,59,60,66-68] thus rendering postoperative RT much less effective. Another study showed that a PSADT of ≥ 5 months predicted a response to SRT (a response was defined as a PSA nadir of ≤ 0.1 ng/mL).[76] One caveat concerning the PSADT as a reliable predictor of distant metastasis is that when the PSA is below 1 ng/mL, the estimates may be inaccurate.[68,77,78] In reports of postoperative RT, few have identified PSADT as a predictor of FFBF. In a preliminary recursive partitioning analysis of 1,168 men in a pooled multi-institutional database, PSADT was not independently related to outcome, although pre-RT PSA, Gleason score, and margin status were related.[79] Standardization is needed both for when the PSADT calculation begins (from the PSA just prior to when an accelerated rise occurs or from the time of the first detectable PSA) and for the minimum number of PSA values required to accurately calculate a PSADT.
The pre-RT PSA has been found to be the most consistent predictor of FFBF in both univariate and multivariate analyses of SRT.[80-83] Although a clear pre-RT PSA cutpoint has not yet been defined, evidence suggests that lower pre-RT PSAs are associated with higher FFBF rates. The best results have been seen when the pre-RT PSA is ≤ 1 ng/mL. A significant decline in FFBF is seen when the pre-RT PSA increases from ≤ 1 ng/mL to 2 ng/mL, and then to > 2 ng/mL. Data suggest that initiating SRT at a lower PSA level leads to an improved outcome, with each incremental 0.1 ng/mL PSA increase resulting in an average 2.6% loss in the percentage of patients with relapse-free survival.[84]
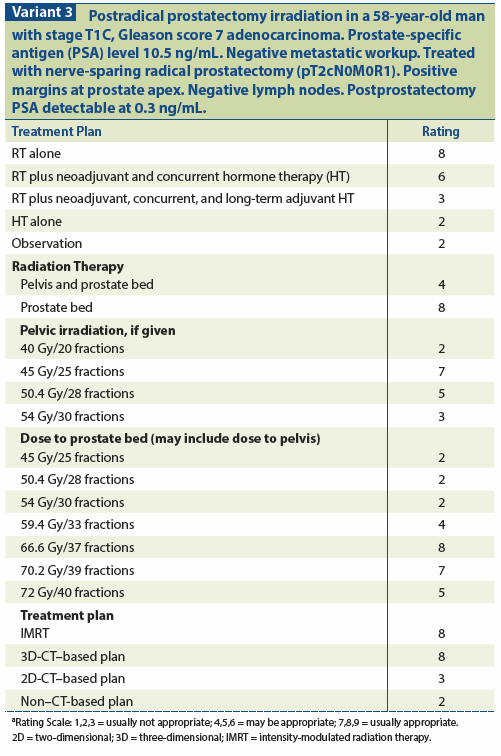
Other important prognostic factors include the Gleason score, margin status, and seminal vesicle invasion. Gleason scores of ≤ 7 predict for a better prognosis compared with scores of 8–10. A positive margin often indicates residual disease in the prostate bed, for which SRT is effective, and FFBF rates are higher when this is the case. Seminal vesicle invasion has also been found to be a determinant of outcome in multivariate analysis in many series, with worse FFBF rates when the seminal vesicles were involved, due to these patients being at a higher risk for the development of subsequent metastatic failure[7,48,49] (see Variant 3).
External Beam Therapy
Intensity-modulated radiation therapy (IMRT) and image-guided RT
External beam therapy is the standard mode of delivery. Multiple studies, as described above, demonstrate its effectiveness. As with definitive external beam therapy for prostate cancer, multiple techniques may be used in the postoperative setting. IMRT or three-dimensional–conformal radiation therapy (3D-CRT) and image guidance are the preferred techniques. Dosimetric studies have been done comparing 3D-CRT vs IMRT in the postoperative setting. IMRT may be the preferred technique, as it may allow for dose escalation with limited toxicity.[85-94] Koontz et al[91] compared 3D-CRT and IMRT for postprostatectomy RT. In their comparison, IMRT reduced the volume of bladder and rectum receiving high radiation doses during treatment. Ost et al[93] evaluated 104 patients using IMRT to a median dose of 74 Gy in the postprostatectomy setting. The toxicity profile was acceptable. Using European Organisation for Research and Treatment of Cancer consensus guidelines for target volumes, Harrison et al[90] compared IMRT (at 72 Gy) vs 3D-CRT (to 68.4 Gy) in 28 patients. The dosimetric parameters were improved with IMRT with respect to dose to the rectum and bladder.
In the SRT setting, in a comparison study of 3D-CRT and IMRT, Goenka et al[88] demonstrated a similar risk of grade > 2 genitourinary (GU) toxicity and a reduction in the risk of grade > 2 gastrointestinal (GI) toxicity. In the salvage setting, with or without androgen deprivation, Ost et al[94] were able to deliver 76 Gy with a toxicity profile similar to that of 3D-CRT delivered at a dose of 68 Gy. In addition, Ost et al[92] did a matched-control analysis of ART and SRT using IMRT. First, for ART the dose was 74 Gy and for SRT the dose was 76 Gy. Secondly, the authors demonstrated a benefit for ART over SRT. Lastly, for ART, the GI and GU toxicity rates were zero and 4%, respectively. In the SRT group, GI and GU toxicity rates were 3% and 3%, respectively. In a large group comparison study, Crandley et al[87] analyzed complications related to IMRT vs 3D-CRT. There has been an increased use of IMRT as the technique of choice. The study showed a decrease in GI complications but a higher rate of GU incontinence with IMRT, compared with 3D-CRT. Nonetheless, a comparison review of IMRT and 3D-CRT by Goldin et al[89] showed no significant difference in rates of long-term GI complications, nonurinary incontinence morbidity, GU incontinence, or erectile dysfunction. Using these comparisons, the bulk of the data favor IMRT over 3D-CRT.
Furthermore, based on the experience gleaned from multiple studies in the setting of treatment for primary intact prostate cancer, IMRT may be considered the technique of choice. Zelefsky et al[95] showed a decrease in rectal toxicity with IMRT compared with 3D-CRT, with a reduction of grade 2/3 rectal bleeding, from 15% with 3D-CRT to 3% with IMRT. In a 2002 report by Zelefsky et al[96] involving 772 patients, the 3-year actuarial rectal grade 2 toxicity was 4%, and the urinary grade 2 toxicity was 15%, comparing favorably with the results of 3D-CRT. In this study, 90% of patients were treated to a dose of 81 Gy, and 10% were treated to 86.4 Gy. The 3-year actuarial PSA biochemical control rates were 92% for favorable disease, 86% for intermediate disease, and 81% for unfavorable disease.
Spratt et al[97] recently updated the Memorial Sloan Kettering experience with prostate cancer patients treated to 86.4 Gy. In this study of 1,002 patients, the 7-year prostate cancer–specific mortality rates were 0%, 3.3%, and 8.1% in the low-risk, intermediate-risk, and high-risk groups, respectively. Rates of Common Terminology Criteria for Adverse Events (CTCAE) v4.0 grade 3 GI and GU toxicity were only 0.7% (mainly rectal bleeding) and 2.2% (mainly urethral strictures and hemorrhagic cystitis), respectively.
Michalski et al[98] recently published in abstract form a re-analysis of the RTOG 9406 dose-escalation studies in which patients were treated to 79.2 Gy using either 3D-CRT or IMRT (in a nonrandomized fashion). The authors found an association between use of IMRT and reduced CTCAE grade 2 or higher acute GU and GI toxicity, although no significant difference in late toxicity was seen.
A study reported by Chung et al[99] also addressed the additional technical improvement of implanting fiducial markers to facilitate image-guided radiation therapy (IGRT). In this study, prostate margins were reduced from 1 cm to 2–3 mm with placement of fiducials, resulting in a decrease in grade 2 rectal toxicity (80% vs 13%, respectively) and bladder toxicity (60% vs 13%, respectively). Zelefsky et al[100] also recently reported on a retrospective series in which men treated to the same dose with IGRT vs IMRT had significantly lower 3-year grade 2+ late urinary toxicity (10.4% vs 20.0%, respectively, P = .03), although no difference in rectal toxicity was seen (P = .81).
Therefore, based on these dosimetric studies and clinical experience, IMRT and image guidance are considered preferable, if not essential, in the delivery of postprostatectomy RT. The appropriate radiation dose to the prostatic fossa in the adjuvant or salvage setting ranges from 64.8 Gy to 70.2 Gy.[11,29-31,101] Higher doses have been delivered with acceptable toxicity[90,93] and may be appropriate under certain conditions.
Summary
- A high percentage of radical prostatectomy (RP) patients with high-risk pathologic features (positive surgical margins, extraprostatic extension of cancer, seminal vesicle involvement) will experience a subsequent biochemical failure, often due to progression of residual disease within the surgical bed.
- The addition of adjuvant RT <br />directed at the prostatic fossa in these patients has been shown in three prospective randomized trials to improve the biochemical freedom-from-failure rate in the irradiated patients and, in one trial, provided improvement in metastasis-free and overall survival.
- Salvage RT, in which patients with biochemically detectable disease receive RT to the prostate bed, has been associated with improvements in cancer-specific and overall survival in retrospective series but has not been tested in a randomized fashion.
- The appropriate radiation dose to the prostatic fossa in the adjuvant or salvage setting ranges from 64.8 Gy to 70.2 Gy. Higher doses may be appropriate if there is evidence of gross recurrence within the prostate bed.
- Addition of pelvic RT to prostatic fossa radiation is generally discouraged, but it may be appropriate in certain clinical situations (eg, absence of lymph node dissection, evidence of nodal involvement at prostatectomy or on imaging studies).
- The benefit of neoadjuvant/adjuvant androgen deprivation therapy with adjuvant or salvage radiation is the subject of ongoing clinical trials.
Androgen Deprivation Therapy
Use of concurrent ADT with ART or SRT may have an impact on the course of the disease by three principal mechanisms: (1) better disease eradication locally (recurrence in a hypoxic scar may be radioresistant), (2) improved disease control distantly (cells in microscopic metastatic deposits might retain sensitivity to ADT), and (3) possible alteration of PSA kinetics by the combination of ADT and RT in patients who eventually relapse.[102,103] The mechanism of the effect on the kinetics of BF and the delayed appearance of distant metastasis is unknown. In some reports,[8,10,22,48,104-111] ADT had positive results in patients at high risk for a rising PSA after SRT (eg, those with a pre-RT PSA > 1 ng/mL).
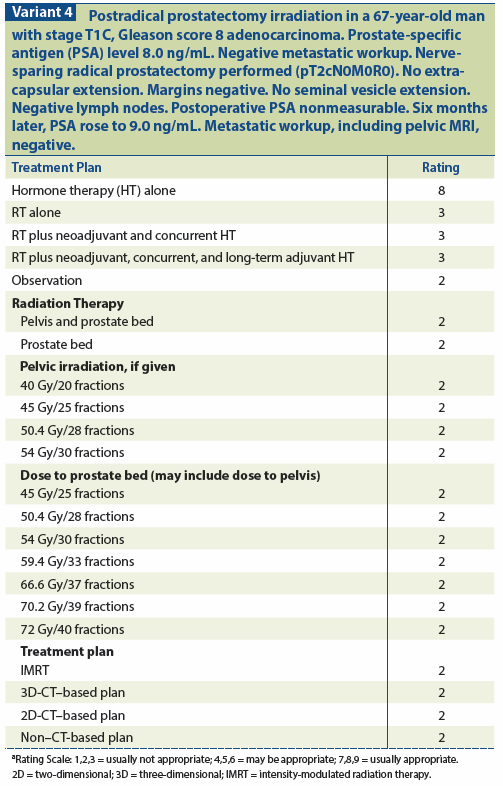
Randomized trials are needed to further evaluate this finding, and are in progress.[112-114] The RTOG 9601 randomized trial has thus far been reported in abstract form (Shipley et al, ASTRO 2010 and ASCO GU 2011), and found that among 771 men with pT3 or margin-positive disease in whom PSA recurrence developed, those randomized to SRT plus 2 years of bicalutamide as opposed to SRT alone had a significantly improved freedom from PSA progression (57% vs 40% at 7 years, P < .0001) and significantly reduced risk of metastases (7.4% vs 12.6%, P = .04). The impact on overall survival awaits further follow-up and more events (see Variant 4).
Adjuvant vs Salvage Radiotherapy
The optimal timing of ART vs SRT for patients with high-risk pathologic features remains controversial.[21,23,24,84,115-117] Presently, there are no published randomized trials comparing ART to planned SRT at established predefined thresholds of BF.[118] Thus, some have supported watchful waiting before administering SRT.[119] The rationale for this approach is based on three points: First, half of all men will be treated unnecessarily. Second, salvage rates are fairly good when the pre-RT PSA is low (≤ 1.0 ng/mL).[69,104,120-122] Third, the progression to distant metastasis after BF may be long.[30,31,59] An important observation is that the addition of SRT in patients who were originally in the observation arm of the SWOG randomized trial still resulted in a higher rate of metastatic failure and reduced overall survival in these patients compared with early adjuvant therapy.[33] Consequently, a recent joint American Urologic Association and American Society of Radiation Oncology guideline supports offering ART to patients with adverse pathologic features.[2,5,15] Similarly, the European Association of Urology developed guidelines on the treatment of advanced, relapsing, and castration-resistant prostate cancer.[3] Without a randomized trial to eliminate selection bias, it is impossible to ascribe an advantage to one strategy over the other based on FFBF outcomes. ART has a proven benefit in randomized prospective studies, supporting first principles that RT treatment should be used if the risk of local failure is > 20% and the side-effect profile is acceptable. Local persistence may lead to the development of distant metastasis in many malignancies. There is evidence that this is the case for prostate cancer.[123-126] In younger men with a long life expectancy and adverse pathologic features, ART should be strongly considered.
Irradiation in Patients With Positive Lymph Nodes
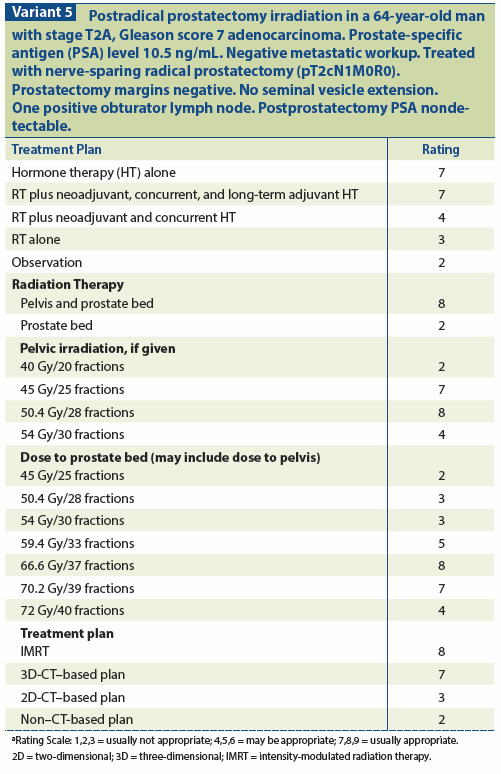
Lymph node involvement (LNI) portends a poor prognosis with a high rate of distant failure. Although there are data indicating that RP or RT should be used along with ADT when LNI is identified,[127] there is no well-established benefit from this approach as yet. ART might be of some value when there is evidence of an appreciable local-regional tumor burden, such as extensive positive margins. There are insufficient data on the subject of pelvic nodal irradiation to make any recommendations, even when LNI has been documented[127-130] (see Variant 5).
The American College of Radiology seeks and encourages collaboration with other organizations on the development of the ACR Appropriateness Criteria through society representation on expert panels. Participation by representatives from collaborating societies on the expert panel does not necessarily imply individual or society endorsement of the final document.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Copyright © 2014 American College of Radiology. Reprinted with permission of the American College of Radiology.
For additional information on ACR Approp-riateness Criteria®, refer to www.acr.org/ac.
References:
1. Bottke D, de Reijke TM, Bartkowiak D, Wiegel T. Salvage radiotherapy in patients with persisting/rising PSA after radical prostatectomy for prostate cancer. Eur J Cancer. 2009;45(Suppl 1):148-57.
2. Valicenti RK, Thompson I, Jr, Albertsen P, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822-8.
3. Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572-83.
4. Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903-7.
5. Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441-9.
6. Choo R, Hruby G, Hong J, et al. (IN)-efficacy of salvage radiotherapy for rising PSA or clinically isolated local recurrence after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2002;53:269-76.
7. Chawla AK, Thakral HK, Zietman AL, Shipley WU. Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: analysis of efficacy and prognostic factors. Urology. 2002;59:726-31.
8. Taylor N, Kelly JF, Kuban DA, et al. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56:755-63.
9. Garg MK, Tekyi-Mensah S, Bolton S, et al. Impact of postprostatectomy prostate-specific antigen nadir on outcomes following salvage radiotherapy. Urology. 1998;51:998-1002.
10. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or persistent PSA after radical prostatectomy. Urology. 2002;60:281-7.
11. Swanson GP, Hussey MA, Tangen CM, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225-9.
12. Kupelian P, Katcher J, Levin H, et al. Correlation of clinical and pathologic factors with rising prostate-specific antigen profiles after radical prostatectomy alone for clinically localized prostate cancer. Urology. 1996;48:249-60.
13. Epstein JI, Partin AW, Sauvageot J, Walsh PC. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol. 1996;20:286-92.
14. Ramos CG, Carvalhal GF, Smith DS, et al. Clinical and pathological characteristics, and recurrence rates of stage T1c versus T2a or T2b prostate cancer. J Urol. 1999;161:1525-9.
15. Gilliland FD, Hoffman RM, Hamilton A, et al. Predicting extracapsular extension of prostate cancer in men treated with radical prostatectomy: results from the population based prostate cancer outcomes study. J Urol. 1999;162:1341-5.
16. Cheng L, Slezak J, Bergstralh EJ, et al. Preoperative prediction of surgical margin status in patients with prostate cancer treated by radical prostatectomy. J Clin Oncol. 2000;18:2862-8.
17. Shah O, Robbins DA, Melamed J, Lepor H. The New York University nerve sparing algorithm decreases the rate of positive surgical margins following radical retropubic prostatectomy. J Urol. 2003;169:2147-52.
18. Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798-803.
19. Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517-23.
20. Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910-4.
21. Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528-34.
22. Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555-65.
23. Oesterling JE, Epstein JI, Walsh PC. Long-term autopsy findings following radical prostatectomy. Urology. 1987;29:584-8.
24. Saleem MD, Sanders H, Abu El Naser M, El-Galley R. Factors predicting cancer detection in biopsy of the prostatic fossa after radical prostatectomy. Urology. 1998;51:283-6.
25. Foster LS, Jajodia P, Fournier G, Jr, et al. The value of prostate specific antigen and transrectal ultrasound guided biopsy in detecting prostatic fossa recurrences following radical prostatectomy. J Urol. 1993;149:1024-8.
26. Naya Y, Okihara K, Evans RB, Babaian RJ. Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology. 2005;66:350-5.
27. Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol. 1994;152:1821-5.
28. Catalona WJ, Smith DS. Cancer recurrence and survival rates after anatomic radical retropubic prostatectomy for prostate cancer: intermediate-term results. J Urol. 1998;160:2428-34.
29. Amling CL, Blute ML, Bergstralh EJ, et al. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101-5.
30. Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591-7.
31. Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433-9.
32. Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32-9.
33. Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow-up of a randomized clinical trial. J Urol. 2009;181:956-62.
34. Paulson DF. Impact of radical prostatectomy in the management of clinically localized disease. J Urol. 1994;152:1826-30.
35. Zietman AL, Edelstein RA, Coen JJ, et al. Radical prostatectomy for adenocarcinoma of the prostate: the influence of preoperative and pathologic findings on biochemical disease-free outcome. Urology. 1994;43:828-33.
36. Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23:651-63.
37. Watson RB, Civantos F, Soloway MS. Positive surgical margins with radical prostatectomy: detailed pathological analysis and prognosis. Urology. 1996;48:80-90.
38. Kupelian PA, Katcher J, Levin HS, Klein EA. Stage T1-2 prostate cancer: a multivariate analysis of factors affecting biochemical and clinical failures after radical prostatectomy. Int J Radiat Oncol Biol Phys. 1997;37:1043-52.
39. Kausik SJ, Blute ML, Sebo TJ, et al. Prognostic significance of positive surgical margins in patients with extraprostatic carcinoma after radical prostatectomy. Cancer. 2002;95:1215-9.
40. Karakiewicz PI, Eastham JA, Graefen M, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245-50.
41. Jones EC. Resection margin status in radical retropubic prostatectomy specimens: relationship to type of operation, tumor size, tumor grade and local tumor extension. J Urol. 1990;144:89-93.
42. Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582-93.
43. Epstein JI, Pound CR, Partin AW, Walsh PC. Disease progression following radical prostatectomy in men with Gleason score 7 tumor. J Urol. 1998;160:97-100.
44. Obek C, Sadek S, Lai S, et al. Positive surgical margins with radical retropubic prostatectomy: anatomic site-specific pathologic analysis and impact on prognosis. Urology. 1999;54:682-8.
45. Emerson RE, Koch MO, Jones TD, et al. The influence of extent of surgical margin positivity on prostate specific antigen recurrence. J Clin Pathol. 2005;58:1028-32.
46. Freedland SJ, Aronson W, Presti JC, Jr, et al. Should a positive surgical margin following radical prostatectomy be pathological stage T2 or T3? Results from the SEARCH database. J Urol. 2003;169:2142-6.
47. Wheeler TM, Dillioglugil O, Kattan MW, et al. Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1-2 prostate cancer. Hum Pathol. 1998;29:856-62.
48. Katz MS, Zelefsky MJ, Venkatraman ES, et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21:483-9.
49. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325-32.
50. Valicenti RK, Gomella LG, Perez CA. Radiation therapy after radical prostatectomy: a review of the issues and options. Semin Radiat Oncol. 2003;13:130-40.
51. Leibovich BC, Engen DE, Patterson DE, et al. Benefit of adjuvant radiation therapy for localized prostate cancer with a positive surgical margin. J Urol. 2000;163:1178-82.
52. Do LV, Do TM, Smith R, Parker RG. Postoperative radiotherapy for carcinoma of the prostate: impact on both local control and distant disease-free survival. Am J Clin Oncol. 2002;25:1-8.
53. Petrovich Z, Lieskovsky G, Stein JP, et al. Comparison of surgery alone with surgery and adjuvant radiotherapy for pT3N0 prostate cancer. BJU Int. 2002;89:604-11.
54. Vargas C, Kestin LL, Weed DW, et al. Improved biochemical outcome with adjuvant radiotherapy after radical prostatectomy for prostate cancer with poor pathologic features. Int J Radiat Oncol Biol Phys. 2005;61:714-24.
55. Lee HM, Solan MJ, Lupinacci P, et al. Long-term outcome of patients with prostate cancer and pathologic seminal vesicle invasion (pT3b): effect of adjuvant radiotherapy. Urology. 2004;64:84-9.
56. Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366:572-8.
57. Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018-27.
58. Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924-30.
59. Jackson WC, Johnson SB, Li D, et al. A prostate-specific antigen doubling time of <6 months is prognostic for metastasis and prostate cancer-specific death for patients receiving salvage radiation therapy post radical prostatectomy. Radiat Oncol. 2013;8:170.
60. D’Amico AV, Moul JW, Carroll PR, et al. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376-83.
61. Ward JF, Blute ML, Slezak J, et al. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170:1872-6.
62. Sengupta S, Myers RP, Slezak JM, et al. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol. 2005;174:2191-6.
63. Patel DA, Presti JC, Jr, McNeal JE, et al. Preoperative PSA velocity is an independent prognostic factor for relapse after radical prostatectomy. J Clin Oncol. 2005;23:6157-62.
64. D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125-35.
65. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760-9.
66. Patel A, Dorey F, Franklin J, deKernion JB. Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. J Urol. 1997;158:1441-5.
67. Leventis AK, Shariat SF, Kattan MW, et al. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19:1030-9.
68. Roberts SG, Blute ML, Bergstralh EJ, et al. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576-81.
69. Brooks JP, Albert PS, Wilder RB, et al. Long-term salvage radiotherapy outcome after radical prostatectomy and relapse predictors. J Urol. 2005;174:2204-8.
70. Nudell DM, Grossfeld GD, Weinberg VK, et al. Radiotherapy after radical prostatectomy: treatment outcomes and failure patterns. Urology. 1999;54:1049-57.
71. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369-75.
72. Mosbacher MR, Schiff PB, Otoole KM, et al. Postprostatectomy salvage radiation therapy for prostate cancer: impact of pathological and biochemical variables and prostate fossa biopsy. Cancer J. 2002;8:242-6.
73. MacDonald OK, Schild SE, Vora SA, et al. Radiotherapy for men with isolated increase in serum prostate specific antigen after radical prostatectomy. J Urol. 2003;170:1833-7.
74. MacDonald OK, Schild SE, Vora S, et al. Salvage radiotherapy for men with isolated rising PSA or locally palpable recurrence after radical prostatectomy: do outcomes differ? Urology. 2004;64:760-4.
75. Peyromaure M, Allouch M, Eschwege F, et al. Salvage radiotherapy for biochemical recurrence after radical prostatectomy: a study of 62 patients. Urology. 2003;62:503-7.
76. Numata K, Azuma K, Hashine K, Sumiyoshi Y. Predictor of response to salvage radiotherapy in patients with PSA recurrence after radical prostatectomy: the usefulness of PSA doubling time. Jpn J Clin Oncol. 2005;35:256-9.
77. Ng MK, Van As N, Thomas K, et al. Prostate-specific antigen (PSA) kinetics in untreated, localized prostate cancer: PSA velocity vs PSA doubling time. BJU Int. 2009;103:872-6.
78. Swindle PW, Kattan MW, Scardino PT. Markers and meaning of primary treatment failure. Urol Clin North Am. 2003;30:377-401.
79. Pollack A, Hanlon AL, Pisansky TM, et al. A multi-institutional analysis of adjuvant and salvage radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2004;60:S186-S7.
80. Forman JD, Meetze K, Pontes E, et al. Therapeutic irradiation for patients with an elevated post-prostatectomy prostate specific antigen level. J Urol. 1997;158:1436-9.
81. Morris MM, Dallow KC, Zietman AL, et al. Adjuvant and salvage irradiation following radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 1997;38:731-6.
82. Rogers R, Grossfeld GD, Roach M 3rd, et al. Radiation therapy for the management of biopsy-proved local recurrence after radical prostatectomy. J Urol. 1998;160:1748-53.
83. Valicenti RK, Gomella LG, Ismail M, et al. Effect of higher radiation dose on biochemical control after radical prostatectomy for PT3N0 prostate cancer. Int J Radiat Oncol Biol Phys. 1998;42:501-6.
84. King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys. 2012;84:104-11.
85. Alongi F, Fiorino C, Cozzarini C, et al. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother Oncol. 2009;93:207-12.
86. Bastasch MD, Teh BS, Mai WY, et al. Post-nerve-sparing prostatectomy, dose-escalated intensity-modulated radiotherapy: effect on erectile function. Int J Radiat Oncol Biol Phys. 2002;54:101-6.
87. Crandley EF, Hegarty SE, Hyslop T, et al. Treatment-related complications of radiation therapy after radical prostatectomy: comparative effectiveness of intensity-modulated versus conformal radiation therapy. Cancer Med. 2014:3:397-405.
88. Goenka A, Magsanoc JM, Pei X, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol. 2011;60:1142-8.
89. Goldin GH, Sheets NC, Meyer AM, et al. Comparative effectiveness of intensity-modulated radiotherapy and conventional conformal radiotherapy in the treatment of prostate cancer after radical prostatectomy. JAMA Intern Med. 2013;173:1136-43.
90. Harrison A, Studenski M, Harvey A, et al. Potential for dose escalation in the postprostatectomy setting with intensity-modulated radiation therapy: a dosimetric study using EORTC consensus guidelines for target volume contours. Pract Radiat Oncol. 2011;1:105-14.
91. Koontz BF, Das S, Temple K, et al. Dosimetric and radiobiologic comparison of 3D conformal versus intensity modulated planning techniques for prostate bed radiotherapy. Medl Dosim. 2009;34:256-60.
92. Ost P, De Troyer B, Fonteyne V, et al. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316-22.
93. Ost P, Fonteyne V, Villeirs G, et al. Adjuvant high-dose intensity-modulated radiotherapy after radical prostatectomy for prostate cancer: clinical results in 104 patients. Eur Urol. 2009;56:669-75.
94. Ost P, Lumen N, Goessaert AS, et al. High-dose salvage intensity-modulated radiotherapy with or without androgen deprivation after radical prostatectomy for rising or persisting prostate-specific antigen: 5-year results. Eur Urol. 2011;60:842-9.
95. Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491-500.
96. Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111-6.
97. Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686-92.
98. Michalski JM, Yan Y, Watkins-Bruner D, et al. Preliminary analysis of 3D-CRT vs IMRT on the high dose arm of the RTOG 0126 prostate cancer trial: toxicity report. Int J Radiat Oncol Biol Phys. 2011;81:S1-S2.
99. Chung HT, Xia P, Chan LW, et al. Does image-guided radiotherapy improve toxicity profile in whole pelvic-treated high-risk prostate cancer? Comparison between IG-IMRT and IMRT. Int J Radiat Oncol Biol Phys. 2009;73:53-60.
100. Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125-9.
101. King CR, Kapp DS. Radiotherapy after prostatectomy: Is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys. 2008;71:346-50.
102. Hanlon AL, Horwitz EM, Hanks GE, Pollack A. Short-term androgen deprivation and PSA doubling time: their association and relationship to disease progression after radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:43-52.
103. Kaminski JM, Hanlon AL, Joon DL, et al. Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys. 2003;57:24-8.
104. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63:134-40.
105. Corn BW, Winter K, Pilepich MV. Does androgen suppression enhance the efficacy of postoperative irradiation? A secondary analysis of RTOG 85-31. Radiation Therapy Oncology Group. Urology. 1999;54:495-502.
106. de la Taille A, Flam TA, Thiounn N, et al. Predictive factors of radiation therapy for patients with prostate specific antigen recurrence after radical prostatectomy. BJU Int. 2002;90:887-92.
107. Jani AB, Sokoloff M, Shalhav A, Stadler W. Androgen ablation adjuvant to postprostatectomy radiotherapy: complication-adjusted number needed to treat analysis. Urology. 2004;64:976-81.
108. King CR, Presti JC, Jr, Gill H, et al. Radiotherapy after radical prostatectomy: Does transient androgen suppression improve outcomes? Int J Radiat Oncol Biol Phys. 2004;59:341-7.
109. Tiguert R, Rigaud J, Lacombe L, et al. Neoadjuvant hormone therapy before salvage radiotherapy for an increasing post-radical prostatectomy serum prostate specific antigen level. J Urol. 2003;170:447-50.
110. Jang JW, Hwang WT, Guzzo TJ, et al. Upfront androgen deprivation therapy with salvage radiation may improve biochemical outcomes in prostate cancer patients with post-prostatectomy rising PSA. Int J Radiat Oncol Biol Phys. 2012;83:1493-9.
111. Soto DE, Passarelli MN, Daignault S, Sandler HM. Concurrent androgen deprivation therapy during salvage prostate radiotherapy improves treatment outcomes in high-risk patients. Int J Radiat Oncol Biol Phys. 2012;82:1227-32.
112. Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040-5.
113. Parker C, Sydes MR, Catton C, et al. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 2007;99:1376-9.
114. Trans-Tasman Radiation Oncology Group (TROG). Radiotherapy-adjuvant versus early salvage. A phase III multi-centre randomised trial comparing adjuvant radiotherapy (RT) with early salvage RT in patients with positive margins or extraprostatic disease following radical prostatectomy. [NCT00860652]. Available from: http://clinicaltrials.gov/ct2/show/record/NCT00860652?term=nct00860652&rank=1. Accessed November 21, 2014.
115. D’Amico AV, Chen MH, Sun L, et al. Adjuvant versus salvage radiation therapy for prostate cancer and the risk of death. BJU Int. 2010;106:1618-22.
116. King CR. Adjuvant versus salvage radiotherapy after prostatectomy: the apple versus the orange. Int J Radiat Oncol Biol Phys. 2012;82:1045-6.
117. King CR. Adjuvant versus salvage radiotherapy for high-risk prostate cancer patients. Semin Radiat Oncol. 2013;23:215-21.
118. Bottke D, Bartkowiak D, Schrader M, Wiegel T. Radiotherapy after radical prostatectomy: immediate or early delayed? Strahlenther Onkol. 2012;188:1096-101.
119. Forman JD, Velasco J. Therapeutic radiation in patients with a rising post-prostatectomy PSA level. Oncology (Williston Park). 1998;12:33-9.
120. Terai A, Matsui Y, Yoshimura K, et al. Salvage radiotherapy for biochemical recurrence after radical prostatectomy. BJU Int. 2005;96:1009-13.
121. Pazona JF, Han M, Hawkins SA, et al. Salvage radiation therapy for prostate specific antigen progression following radical prostatectomy: 10-year outcome estimates. J Urol. 2005;174:1282-6.
122. Choo R, Morton G, Danjoux C, et al. Limited efficacy of salvage radiotherapy for biopsy confirmed or clinically palpable local recurrence of prostate carcinoma after surgery. Radiother Oncol. 2005;74:163-7.
123. Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199-205.
124. Zagars GK, von Eschenbach AC, Ayala AG, et al. The influence of local control on metastatic dissemination of prostate cancer treated by external beam megavoltage radiation therapy. Cancer. 1991;68:2370-7.
125. Kuban DA, el-Mahdi AM, Schellhammer PF. Effect of local tumor control on distant metastasis and survival in prostatic adenocarcinoma. Urology. 1987;30:420-6.
126. Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;21:537-47.
127. Pollack A, Horwitz EM, Movsas B. Treatment of prostate cancer with regional lymph node (N1) metastasis. Semin Radiat Oncol. 2003;13:121-9.
128. Cheng CW, Bergstralh EJ, Zincke H. Stage D1 prostate cancer. A nonrandomized comparison of conservative treatment options versus radical prostatectomy. Cancer. 1993;71:996-1004.
129. Galalae RM, Kovacs G, Schultze J, et al. Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52:81-90.
130. Rusthoven CG, Carlson JA, Waxweiler TV, et al. The impact of definitive local therapy for lymph node-positive prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2014;88:1064-73.