Prostate cancer is the most common malignancy affecting men. There has been a nearly 70% increase in new prostate cancer cases, mostly classified as low risk, that have been diagnosed in early stages as a consequence of prostate-specific antigen (PSA) screening. Data regarding the natural history of this disease confirm the clinical insignificance of low-grade prostate cancer, which is associated with scant or no metastatic dissemination. Active surveillance is a conservative management approach, conducted for those patients with “low-risk” or “favorable-risk” disease, which avoids long-term adverse effects on the patient’s quality of life. It is characterized by a routine protocol of close monitoring with digital rectal examination, periodic biopsy, and serial PSA testing. As defined by D’Amico, active surveillance is broadly appropriate for men with a Gleason score of 6 or less and a PSA level of less than 10 ng/mL. Typically, Gleason pattern 3 disease lacks the common genetic aberrancies of a true cancer. An essential element of the active surveillance approach is early recognition of higher-risk disease, which is diagnosed by systematic biopsy in 30% of patients who initiate active surveillance with low-risk disease. Also, a small group of patients have molecular alterations that can cause progression to more aggressive disease; these men can be switched to immediate treatment if such progression is detected. Oncologic outcomes for active surveillance cohorts have shown the long-term safety of this approach, with a cancer-specific mortality rate of 3% at 10 to 15 years. In this review of active surveillance for favorable-risk prostate cancer, we will discuss the rationality of this approach, the biological evidence for employing active surveillance in Gleason pattern 3 and 4 prostate cancer, patient selection for active surveillance, clinical trial data on active surveillance, and the role of prostate cancer biomarkers and imaging studies (MRI) for clinical decision making in patients with low-risk disease.
Introduction
According to the American Cancer Society, in 2016 an estimated 180,890 new cases of prostate cancer were diagnosed in the United States, making this disease the most common solid tumor in men.[1] Despite the high incidence, only 26,120 men are estimated to have died of prostate cancer in 2016; the 10-year and 15-year relative survival rates for prostate cancer are 98% and 95%, respectively.[1] Since the 1980s,[2] widespread screening with serum prostate-specific antigen (PSA) levels and digital rectal examination (DRE) have facilitated early detection, and the incidence of prostate cancer has increased dramatically. Almost 80% of cases are detected at clinically localized stage III,[3] and more than half are expected to be low-risk tumors. Such tumors are an infrequent cause of death, and the men affected are more likely to die of other causes. The initial dilemma in the management of clinically localized prostate cancer stems from prostate cancer’s heterogeneity, as evidenced by its natural history. Radical prostatectomy and radiation therapy, the standard treatments for prostate cancer, both frequently result in significant adverse events, including urinary and erectile dysfunction. Many men who receive active treatment do not derive any clinical benefit from their treatment, due to the slow progression of early-stage, low-risk cancer.
Active surveillance is an approach whose intention is to diminish the morbidities of immediate active treatment for men with low-risk prostate cancer who likely will never develop cancer-related symptoms. It was first described in 2002 in a report of 206 patients managed with periodic prostate biopsies and serial PSA testing, with radical intervention recommended for patients reclassified as higher risk.[4] The active surveillance strategy aimed to diagnose, observe, and act with the intention to cure only when essential. After 20 years of experience with this approach, active surveillance has become widely adopted around the world.
Background and Rationale
Prostate cancer screening using serum PSA levels was first reported by Catalona in 1991.[3] The rapid adoption of PSA screening in North America and Europe dramatically increased the incidence of prostate cancer that was identified, much of which was microfocal low-grade disease. This epidemiologic phenomenon resulted in the overdetection and overtreatment of insignificant disease, raising the risk of unnecessary morbidity and impairment of men’s health-related quality of life.[5] Over the following 10 years, more than 90% of patients with low-risk prostate cancer were managed with radical therapies (radical prostatectomy or radiation). Overdetection trends were sustained until 2012, when the United States Preventive Services Task Force (USPSTF) assigned a grade of “D” to the use of PSA-based screening for prostate cancer (meaning that they recommended against the practice) for men of all ages (the USPSTF had already recommended against PSA-based screening in men aged ≥ 75 years in 2008).[6] Subsequent to this recommendation, rates of PSA screening decreased by 3% to 10% in all age groups, followed by a shift toward detection of tumors of higher grade and stage.[7] Furthermore, solid evidence regarding the biology of low-grade prostate cancer established the indolent nature of the disease. This knowledge resulted in a reexamination of the role of radical intervention for low-risk cancer.
Active surveillance has emerged as a conservative approach that can mitigate overtreatment. Current practice protocols combine clinical T stage, PSA value, PSA density, Gleason score, number of cores involved (based on 12-core biopsies), and amount of malignancy per core in order to select patients for active surveillance and predict the risk of occult coexistent higher-grade cancer. Data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry demonstrates the increase in the use of active surveillance for patients with low-risk prostate cancer-from a low of 6.7% in the years from 1990 to 2009, to 40.4% in the period 2010 to 2013.[8] Active surveillance has emerged as a conservative approach that can mitigate overtreatment (Figure).
Natural History and Genetic Features of Low-Grade Prostate Cancer
The chief dilemma in managing clinically localized prostate cancer stems from the heterogeneity of the disease. Prostate cancer arises from genetically altered prostate epithelium and slowly progresses over several decades.[9] Given its features of multifocality and tumor heterogeneity, the course of prostate cancer is difficult to predict. Men may live their entire natural life without having any symptoms from prostate cancer. Zlotta et al[10] confirmed this hypothesis when they prospectively compared tissue obtained during autopsy from prostate glands in both a Caucasian and an Asian population. Prostate cancer was found in a similar proportion (35%) of men in both groups. Also, more than 50% of the cancers in the Asian group had a Gleason score of 7 or greater. The natural history of this disease, which is characterized by slow progression, makes it possible for active surveillance to be an effective management strategy.
Gleason patterns 3 and 4 are dramatically different genetically. The hallmarks of cancer include six biological capabilities that are typically acquired by human tumors during their development: 1) sustained proliferative signaling, 2) activation of local invasion and metastasis, 3) induction of angiogenesis, 4) evasion of growth suppressors, 5) resistance to cell death, and 6) unlimited replicative potential.[11] Ahmed et al[12] applied these oncologic principles in a review that described the different features of Gleason patterns 3 and 4, and concluded that most small Gleason pattern ≤ 3 lesions could be considered nonmalignant. Examples of the differences between Gleason 3 and Gleason 4 lesions include the findings that translocation of TMPRSS2-ERG,[13] PTEN deletion,[14] sustained vascular endothelial growth factor–induced angiogenesis,[15] and resistance to apoptosis resulting from strong DAD1 expression[16] are most common in Gleason pattern 4 lesions, and are absent or present only at low levels (10%) in Gleason pattern 3 lesions. Clearly, solid molecular evidence suggests that Gleason pattern 3 (or Gleason 3+3=6) disease lacks the hallmarks of cancer, as defined in terms of gene expression abnormalities-and as backed up by clinical experience. The molecular and histologic patterns of Gleason 3 lesions are likely predetermined to remain stable or even involute. In contrast, Gleason pattern 4 lesions exhibit most of the molecular characteristics of cancer.
A number of large surgical series have reported a rate of metastasis that approximates zero in surgically confirmed Gleason 6 prostate cancer. In order to understand the natural history of surgically treated Gleason 6 prostate cancer, Eggener et al[17] conducted a multicenter study of 24,000 men with long-term follow-up. Of those men, 12,000 had confirmed Gleason 6 disease. The 15-year prostate cancer–specific mortality rate for pathologic Gleason 6 disease was 0.2%. Only 1 patient in the cohort died of prostate cancer; a pathology review reported Gleason 4+3 disease in this man instead of what was initially thought to be Gleason 3+3=6 disease. An additional series from the Hopkins pathology group examined cases with a Gleason score of ≤ 6 from radical prostatectomy databases from four large academic centers. A total of 14,123 cases were identified, which altogether showed 22 lymph node metastases. A new histopathology review of those 22 lymph nodes reported not a single case of a tumor with a Gleason score of ≤ 6 that was associated with lymph node metastasis.[18]
The key point here is that most Gleason 6 cancers have innocent genetic features and no risk of metastasis. Thus, in the absence of higher-grade cancer, there is little indication for treatment in most patients.
Outcomes of Active Surveillance
Observational studies
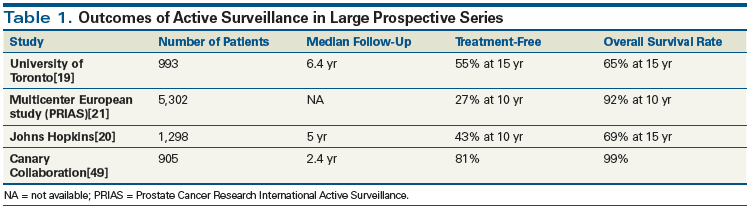
Multiple groups worldwide have reported the results of prospective cohorts that provide data about the clinical course of patients managed with active surveillance. Data and outcomes from those groups (summarized in Table 1) consistently show a low rate of progression to metastatic disease or death from prostate cancer. The first study of active surveillance was started by researchers at the University of Toronto in 1996.[19] This single-arm cohort study included 993 men, 740 with low-risk disease (Gleason score ≤ 6 and serum PSA level ≤ 10 ng/mL) and 253 with intermediate-risk disease (serum PSA ≤ 15 ng/mL or a Gleason score of 7 [3+4]) who had significant comorbidity or a life expectancy of less than 10 years. Among the 819 survivors, the median follow-up was 6.4 years. Altogether, 2.8% of the patients developed metastatic disease, and 1.5% died of prostate cancer. In the group with low-risk disease, the metastasis-free survival rate at 10 and 15 years was 96% and 95%, respectively. In the group with intermediate-risk disease, the metastasis-free survival rate was 91% and 82% at 10 and 15 years, respectively. Importantly, the presence of Gleason pattern 4 disease at diagnosis increased the rate of metastasis by 3.75, despite close monitoring and treatment for progression.
By comparison, researchers at Johns Hopkins have limited surveillance strictly to patients who fulfilled the Epstein criteria (≤ 2 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL/cm3).[20] A total of 1,298 men were included in this cohort; the median follow-up was 5 years. Overall survival, cancer-specific survival, and metastasis-free survival rates were 94%, 99.9%, and 99.4%, respectively, at 10 years; these rates were 69%, 99.9%, and 99.4%, respectively, at 15 years. Not surprisingly, the 15-year prostate cancer–specific mortality rate was only 0.4%. These excellent results are explained by the more rigorous active surveillance eligibility criteria used by this group.
Recently, the Prostate Cancer Research International Active Surveillance (PRIAS) study reported data after 10 years of follow-up.[21] This multicenter international cohort included 5,302 patients with low-risk prostate cancer managed with active surveillance. At 5 and 10 years of follow-up, 52% and 73%, respectively, had discontinued active surveillance. Of those men who discontinued active surveillance, 62% were reclassified as higher risk. A third of men had subsequent surgical intervention (radical prostatectomy) and were found, despite the decision to intervene, to have favorable pathologic tumor features (Gleason 3+3 and pT2). Gleason upgrading (to Gleason score > 7) or clinical T3 disease was the only protocol-based indication for active treatment.
Randomized trials
Three trials have been published that investigated the effectiveness of immediate treatment (radical prostatectomy) vs active surveillance for patients with localized prostate cancer detected by PSA screening. The Prostate Cancer Intervention vs Observation Trial (PIVOT) randomly assigned 731 men with prostate cancer to radical prostatectomy or observation. During the median follow-up of 12 years, the group treated by surgical intervention (47%) did not exhibit a significantly reduced all-cause or prostate cancer–specific mortality rate, as compared with the observation group (49%).[22] The second of these large studies was the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) randomized clinical trial. This prospective study compared radical prostatectomy vs watchful waiting in early prostate cancer. After 18 years of follow-up, overall mortality and prostate cancer–specific mortality rates were higher in patients managed with watchful waiting than in those who received immediate treatment.[23] Unfortunately, neither PIVOT nor SPCG-4 used an active surveillance approach: patients received treatment if there was evidence of progression to metastatic disease. In contrast, men on active surveillance are closely followed, rebiopsied, and treated if there is evidence of grade progression or risk reclassification-in order to prevent metastatic progression.
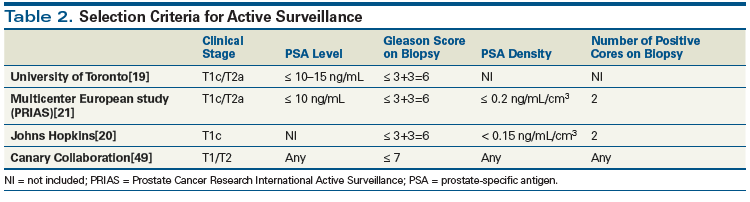
Lastly, the Prostate Testing for Cancer and Treatment (ProtecT) trial compared three modalities of management-active monitoring, radical prostatectomy, and external-beam radiotherapy-in patients with localized prostate cancer.[24] Patients randomized to active monitoring had their PSA level measured every 3 months during the first year and every 6 to 12 months thereafter. Among the 2,664 patients with a diagnosis of prostate cancer, there were 17 prostate cancer–specific deaths overall (8, 5, and 4 in the active monitoring, surgery, and radiotherapy groups, respectively), demonstrating there was no significant difference in the 10-year cancer-specific survival rate or the overall survival rate. There was a difference in the metastasis rate favoring radical treatment. This likely reflects the fact that 25% of the cohort had intermediate- or high-risk disease, for which conservative management is clearly associated with an increased risk of progression. Further, “active monitoring,” while more intensive than “watchful waiting,” did not include serial biopsies or predefined indications for intervention.
Patient Selection
The main concern in selecting patients for long-term conservative management is to identify those with occult coexistent higher-grade cancer.[25] Currently, definitive criteria for the selection of patients for active surveillance are lacking. Large, prospective, single-institution cohorts have provided the basis for guidelines for the identification of patients at low risk for disease progression (Table 2).
The Toronto group also has reported data on active surveillance for patients with intermediate-risk prostate cancer. Klotz et al offered active surveillance to some men with a Gleason score ≤ 3+4 and/or a PSA level of 10 to 20 ng/mL, and with a life expectancy of less than 10 years.[26] The initial cohort showed that in this group of men with intermediate-risk prostate cancer, only 1 patient out of 85 experienced progression to metastatic disease. Further investigation is needed to determine progression factors in this subgroup of patients with Gleason 7 prostate cancer.
Because of the very favorable experience with active surveillance in Gleason 6 cancer, and the significantly higher rate of progression seen with Gleason 7 disease, most groups now take a middle-of-the-road approach, offering active surveillance to most patients with Gleason 6 disease regardless of cancer volume, and being very selective about offering it to patients with Gleason 7 disease.
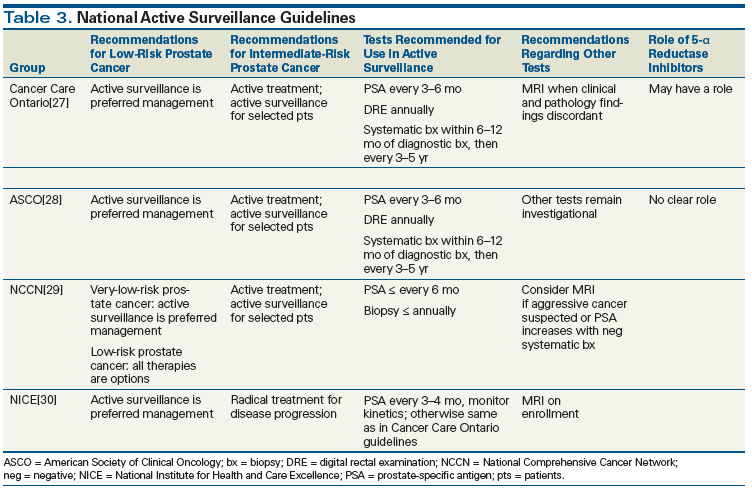
Guidelines have been developed by many groups, including Cancer Care Ontario,[27] the American Society of Clinical Oncology (ASCO),[28] the National Comprehensive Cancer Network (NCCN),[29] and the National Institute for Health and Care Excellence (NICE; United Kingdom).[30] These are summarized in Table 3. There are large areas of agreement among these sets of guidelines, and they concur that surveillance should be the preferred strategy for most low-risk patients. There are variations in eligibility criteria and follow-up strategy, however. For example, the Johns Hopkins program used stringent eligibility criteria, according to which inclusion is based on the Epstein criteria for clinically insignificant prostate cancer (PSA density < 0.15 ng/mL/cm3, Gleason score ≤ 6, ≤ 2 positive biopsy cores, and ≤ 50% involvement of any biopsy core). Reflecting this approach, the NCCN guidelines make a distinction between very-low-risk disease (defined as fulfilling the Epstein criteria), for which active surveillance is the recommended strategy, and low-risk disease (Gleason score of 6 but higher volume of cancer on biopsy), for which all therapies, including surgery and radiation, are considered options. In contrast, according to the Cancer Care Ontario and ASCO guidelines, all men with low-risk prostate cancer are candidates for surveillance.[27,28]
Risk factors for progression beyond biopsy features
PSA density. A high PSA density has been associated with an increased risk of biopsy reclassification in multiple studies.[31] Essentially this means that patients with a high PSA level relative to their prostate volume are at increased risk, and should be scrutinized very carefully before being placed on surveillance. In contrast, those with a large prostate volume relative to their PSA level can be reassured that they are less likely to harbor aggressive disease.
Impact of race. Several studies have reported higher rates of occult coexisting cancer in African-American patients who meet criteria for low-risk disease, as well as higher rates of biochemical recurrence after surgery.[32-34] However, a recent large study of the Shared Equal Access Regional Cancer Hospital (SEARCH) database, which included 355 African-American men and 540 Caucasian men with low-risk prostate cancer, showed no difference in pathologic upstaging or biochemical recurrence between the two groups, reinforcing the appropriateness of surveillance for African-American men.[35]
Impact of age. Young age has not been demonstrated to be a risk factor for progression. Indeed, the likelihood of harboring higher-grade cancer at diagnosis is lower in young men with Gleason 6 disease. In the Sakr autopsy series, 29% of men in their 30s harbored microfocal Gleason 6 cancer.[36] Finding small amounts of low-grade cancer in a young man in no way means that his disease is destined to progress to significant cancer. The caveat is that younger patients have a longer period of time in which to develop biological progression to higher-grade cancer. The best estimate of the likelihood of grade progression (as distinct from disease reclassification due to more accurate or repeat sampling) is 1.2% to 2% per year.[37] A recent study evaluated the association of age with various active surveillance endpoints.[38] Patients less than 60 years old received more surveillance biopsies per time interval. Despite the more intensive assessment, younger age was associated with a lower risk of biopsy-based upgrade and progression. There were no differences with respect to risk of definitive treatment or risk of biochemical recurrence after delayed radical prostatectomy. Younger patients should be advised that intermediate-term outcomes are not worse, but that longer-term follow-up is needed. Thus, young patients can in most cases be managed conservatively but require long-term follow-up.
Active Surveillance Follow-Up
Active surveillance protocols have evolved in recent years. There are variations between the protocols of different institutions, and a standardized protocol does not yet exist. The principle modality of active surveillance follow-up is a combination of serial PSA measurements and DREs. In a typical clinical scenario, a patient comes to clinic with an initial diagnosis of Gleason 6 prostate cancer on a systematic prostate biopsy (10 or more cores). Regular follow-up is conducted with PSA measurement every 6 months and annual DRE. In addition, a confirmatory biopsy is ordered within 6 to 12 months of the initial diagnostic biopsy. This confirmatory biopsy should target the areas that are often missed on systematic diagnostic biopsies. If the confirmatory biopsy is either negative or confirms microfocal Gleason 6 disease, subsequent biopsies are performed every 3 to 5 years. We stop subsequent biopsies when the patient reaches the age of 80 or has a life expectancy of < 5 years. In those patients with an increase in prostate cancer volume or a new biopsy showing Gleason 3+4 disease but who still desire surveillance as a management option, and in those whose PSA doubling time is < 3 years, a multiparametric MRI should be performed. Identification of any suspicious lesion with restricted diffusion on the MRI should lead to a targeted biopsy.
The Role of MRI and Biomarkers
Key Points
- Gleason pattern 3 disease (Gleason score 6, now known as Gleason grade group 1) does not metastasize. In their molecular genetics, most Gleason pattern 3 cells resemble normal cells. In contrast, Gleason pattern 4 cells in most cases have the hallmarks of malignancy.
- High-volume pattern 3 disease is significant, not because it poses a threat to the patient, but because it is associated with an increased risk of coexistent higher-grade cancer. Thus, such patients require closer scrutiny, but don't need to be treated unless higher-grade cancer is identified.
- Any Gleason pattern 4 disease at baseline is associated with a significant increase in the risk of progression to metastatic disease. In the Toronto cohort, the patients with Gleason 3+4=7 disease at baseline had a 3.8× greater risk of metastasis at 15 years (20% vs 5%). Thus, patients with Gleason 7 cancer and a > 10–15-year life expectancy should in most cases be treated.
- Multiparametric MRI and biomarkers will likely expand the indications for surveillance-by identifying intermediate-risk patients with favorable-risk disease, and by reassuring low-risk patients that they are not harboring higher-risk cancer.
An unmet need among physicians and patients using the active surveillance approach has been a means of avoiding the 25% to 30% risk of misclassification inherent in a systematic biopsy–based diagnostic strategy. There is extensive ongoing research in serum, urinary, and histopathology markers-and imaging studies-that might improve prediction of the natural history of individual cases of prostate cancer.[39]
Since 1982, MRI has been used to evaluate prostate anatomy and disease.[40] A combination of conventional anatomical and functional MRI is known as multiparametric MRI. This imaging study has shown to more accurately identify those patients being managed with active surveillance who have occult coexistent higher–Gleason score cancer. Pessoa et al[41] published the results of a prospective cohort study that enrolled 105 patients with low-risk, low-grade, localized prostate cancer who were candidates for active surveillance; the men subsequently underwent multiparametric MRI. The multiparametric MRI results demonstrated a sensitivity, specificity, positive predictive value, and negative predictive value for disease reclassification of 92.5%, 76%, 81%, and 90.5%, respectively. The high negative predictive value is particularly important in this population, and this figure has been confirmed by many groups. Multiparametric MRI is becoming part of the management of patients with localized prostate cancer as a result of these data.[42]
Similarly, advances in genetic analysis have led to the discovery of new biomarkers that may predict outcomes of prostate cancer and response to therapy. As of the publication of this article, the three genetic tissue assays described below have been cleared by the US Food and Drug Administration for use in men with prostate cancer. However, none of these tests has yet been validated as providing substantial benefit in the active surveillance population.
Genomic classifier
This is a 22-marker genomic test that is based on RNA expression and that utilizes tissue from a prostate biopsy. The genomic classifier test had an independent predictive value on multivariable analysis for predicting metastasis following prostatectomy, with a hazard ratio (HR) of 1.5 for each 10% increase in score; these results were validated in two separate prostatectomy cohorts. A high score is associated with an increased risk of metastasis (HR, 1.7 for each 10% increase in score).[43,44]
Genomic prostate score
This assay, which also utilizes prostate biopsy specimens, incorporates 12 cancer genes that represent four biological pathways of prostate cancer oncogenesis: the androgen receptor pathway, cellular organization, stromal response, and proliferation. A 20-point increase in the “genomic prostate score” is associated with a statistically significant increased risk of high-grade and/or non–organ-confined disease (odds ratio, 1.9; 95% CI, 1.3–2.9).[45,46]
Cell cycle progression
This assay analyzes 31 cell cycle–related genes and 15 housekeeping genes by quantitative reverse transcriptase polymerase chain reaction and generates a “cell cycle progression score.” The Transatlantic Prostate Group examined cell cycle progression scores using needle biopsies of a conservatively managed prostate cancer cohort from Great Britain. In this cohort of 349 men managed without primary treatment, the cumulative incidence of death was increased among those with cell cycle progression scores > 2 (19% of the population) compared with those with lower scores. Patient outcomes could not be differentiated in those who had lower cell cycle progression scores. The HR of prostate cancer death was 1.7 per unit increase in cell cycle progression score.[47,48]
Evidence indicates that these genomic assays can detect the presence of molecular alterations associated with higher-grade cancer on biopsies that demonstrate microfocal Gleason 6 disease. For example, a patient with low-grade prostate cancer, an elevated PSA level, and a positive result on either a genomic classifier or genomic prostate score test should have a multiparametric MRI to rule out any multifocal disease, and may be advised to have radical treatment even if the MRI is not confirmatory. Future research goals will be to integrate and correlate information obtained through multiparametric MRI and results of genomic biomarker assays.
The Future of Active Surveillance
While confidence in conservative management for low-risk disease has increased significantly as the cohorts have matured, many unanswered questions remain. These include the following:
• What are the molecular events that signal progression of low-grade disease?
• How can we optimally identify the “wolves in sheep’s clothing”-ie, those low-grade cases that harbor higher-grade cancer?
• What is the effect of germline genetic alterations-eg, BRCA1/2 mutations-on eligibility for surveillance?
• How should multiparametric MRI and biomarkers be integrated into treatment decision making?
• Which intermediate-risk patients are candidates for surveillance?
• What interventions (diet, exercise, micronutrients, pharmacologic agents) are able to reduce the risk of biological progression in men on active surveillance, and thus are warranted?
• What is the most efficient and cost-effective way to follow patients longitudinally? Is serial biopsy still required, and in whom?
• Can risk stratification allow some patients to minimize the burden of follow-up?
• Can the widespread adoption of surveillance for low-risk disease rehabilitate prostate cancer screening?
Conclusion
PSA screening caused an increase in newly diagnosed cases of low-risk prostate cancer, leading to overtreatment. Clinical and molecular data support the absence of a metastatic phenotype for Gleason pattern 3 cancers. Active surveillance now represents the primary treatment recommended in evidence-based guidelines for most men with low-risk prostate cancer. Implementation of active surveillance improves quality of life compared with radical treatment. Active surveillance should be offered to patients with a low risk of cancer progression, including those with a life expectancy of more than 10 years, clinical stage T1/2a disease, a PSA level < 15 ng/mL, and biopsy Gleason score of ≤ 6.
Thirty percent of patients with newly diagnosed low-risk prostate cancer have an occult higher-grade cancer. A confirmatory biopsy is mandatory within 6 to 12 months after the initial biopsy to exclude any upgraded disease. Long-term monitoring is based on PSA measurement and DRE every 6 months, with successive prostate biopsies and/or MRI every 3 to 5 years. New technologies, such as multiparametric MRI and genomic biomarker assays, should complement the initial assessment of candidates for active surveillance in order to identify aggressive occult cancers and detect progression of disease during follow-up. Radical treatment should be offered to most patients with upgraded disease.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Accessed April 27, 2017.
2. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-61.
3. Han M, Partin AW, Piantadosi S, et al. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416-9.
4. Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664-9.
5. Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies in localized prostate cancer. J Clin Oncol. 2002;20:557-66.
6. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality. 2011 Oct. Report No.:12-05160-EF-1.
7. Fleshner K, Carlsoon SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14:26-37.
8. Tosoian JJ, Carter HB, Lepor A, Loeb S. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol. 2016;13:205-15.
9. Ritmaster RS. 5 α–reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22:389-402.
10. Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013;105:1050-8.
11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74.
12. Ahmed H, Arya M, Emberton M, et al. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012;13:e509-e517.
13. Berg KD, Vainer B, Thomsen FB, et al. ERG protein expression in diagnostic specimens is associated with increased risk of progression during active surveillance for prostate cancer. Eur Urol. 2014;66:851-60.
14. Lotan TL, Carvalho FL, Peskoe SB, et al. PTEN loss is associated with upgrading of prostate cancer from biopsy to radical prostatectomy. Mod Pathol. 2015;28:128-37.
15. West AF, O’Donnell M, Charlton RG, et al. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer. 2001;85:576-83.
16. Guo Y, Sklar GN, Borkowski A, et al. Loss of the cyclin-dependent kinase inhibitor p27 (Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res. 1997;3:2269-74.
17. Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869-75.
18. Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score <6 have the potential to metastasize to lymph nodes? Am J Surg Pathol. 2012;36:1346-52.
19. Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272-7.
20. Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from prospective active surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379-85.
21. Bokhorst LP, Valdagni R, Rannikko A, et al. A decade of active surveillance in the PRIAS Study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70:954-60.
22. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203-13.
23. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932-42.
24. Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-24.
25. Lee SE, Kim DS, Lee WK, et al. Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int. 2010;105:1526-30.
26. Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126-31.
27. Morash C, Tey R, Agbassi C, et al. Active surveillance for the management of localized prostate cancer. Can Urol Assoc J. 2015;9:171-8.
28. Chen RC, Rumble RB, Loblaw DA, et al. Active surveillance for the management of localized prostate cancer (Cancer Care Ontario guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34:2182-90.
29. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Prostate cancer. Version 2.2017. https://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. Accessed April 17, 2017.
30. National Institute for Health and Care Excellence. Prostate cancer: protocol for active surveillance. https://www.nice.org.uk/guidance/cg175/resources/protocol-for-active-surveillance-19674477. Accessed April 17, 2017.
31. Welty CJ, Cowan JE, Nguyen H, et al. Extended follow-up and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193:807-11.
32. Sundi D, Ross AE, Humphreys EB, et al. African American men with very low risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991-7.
33. Sundi D, Faisal FA, Trock BJ, et al. Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology. 2015;85:155-60.
34. Jalloh M, Myers F, Cowan JE, et al. Racial variation in prostate cancer upgrading and upstaging among men with low-risk clinical characteristics. Eur Urol. 2015;67:451-7.
35. Leapman MS, Freedland SJ, Aronson WJ, et al. Pathological and biochemical outcomes among African-American and Caucasian men with low risk prostate cancer in the SEARCH database: implications for active surveillance candidacy. J Urol. 2016;196:1408-14.
36. Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo. 1994;8:439-43.
37. Inoue LY, Trock BJ, Partin AW, et al. Modeling grade progression in an active surveillance study. Stat Med. 2014;33:930-9.
38. Leapman MS, Cowan JE, Nguyen HG, et al. Active surveillance in younger men with prostate cancer. J Clin Oncol. 2017 Mar 27. [Epub ahead of print]
39. van den Bergh RC, Ahmed HU, Bangma CH, et al. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systematic review. Eur Urol. 2014;65:1023-31.
40. Steyn JH, Smith FW. Nuclear magnetic resonance (NMR) imaging of the prostate. Br J Urol. 1982;54:679-81.
41. Pessoa RR, Viana PC, Mattedi RL, et al. Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance. BJU Int. 2016;119:535-42.
42. Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627-36.
43. Klein EA, Haddad Z, Yousefi K, et al. Decipher genomic classifier measured on prostate biopsy predicts metastasis risk. Urology. 2016;90:148-52.
44. Nguyen PL, Martin NE, Choeurng V, et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2017 Jan 24. [Epub ahead of print]
45. Cullen J, Rosner IL, Brand TC, et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur Urol. 2015;68:123-31.
46. Brand TC, Zhang N, Crager MR, et al. Patient-specific meta-analysis of 2 clinical validation studies to predict pathologic outcomes in prostate cancer using the 17-gene genomic prostate score. Urology. 2016;89:69-75.
47. Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106:1095-9.
48. Cuzick J, Stone S, Fisher G, et al. Validation of an RNA cell cycle progression score for predicting death from prostate cancer in a conservatively managed needle biopsy cohort. Br J Cancer. 2015;113:382-9.
49. Newcomb LF, Thompson IM Jr, Boyer HD, et al; Canary PASS Investigators. Outcomes of active surveillance for clinically localized prostate cancer in the prospective, multi-institutional Canary PASS Cohort. J Urol. 2016;195:313-20.