Adjuvant Chemotherapy Improves Survival for Stage II and III NSCLC, but Not for Stage IB, Studies Suggest
Use of adjuvant chemotherapy for stage IB non-small-cell lung cancer (NSCLC) is less certain than it appeared to be at last year's American Society of Clinical Oncology (ASCO) meeting, according to a study reported at the 2006 ASCO Annual Meeting.
ATLANTAUse of adjuvant chemotherapy for stage IB non-small-cell lung cancer (NSCLC) is less certain than it appeared to be at last year's American Society of Clinical Oncology (ASCO) meeting, according to a study reported at the 2006 ASCO Annual Meeting. A second study showed that adjuvant chemotherapy is effective for stage II-III NSCLC and that vinorelbine (Navelbine)/ cisplatin may be better than other doublets. A third study showed that vinorelbine/cisplatin is effective for elderly patients.
CALGB 9633 Update
Gary M. Strauss, MD, MPH, reported updated data from the Cancer and Leukemia Group B (CALGB) 9633 study of adjuvant chemotherapy in stage IB NSCLC (abstract 7007). Preliminary results of that trial reported in 2004 showed significantly better disease-free survival (DFS) and overall survival (OS) with adjuvant carboplatin and paclitaxel. Dr. Strauss said that the study was initially planned to accrue 500 patients but due to the interim analysis and slow accrual, the accrual target was reduced to 384.
Patients were randomized following resection to paclitaxel 200 mg/m2 and carboplatin AUC 6 every 3 weeks for four cycles or to observation. The primary endpoint was overall survival. Dr. Strauss reported data for 344 patients included in the intent-to-treat analysis. Median follow-up was 57 months, and mean tumor size was 4.5 cm.
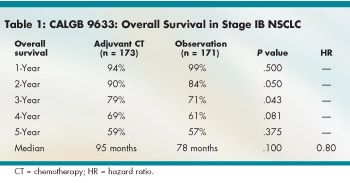
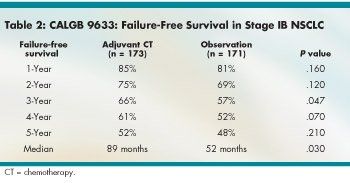
Overall survival was significantly better with adjuvant chemotherapy for only a limited time (see Table 1). The hazard ratio (HR) for median OS was a nonsignificant 0.80. Median failure-free survival (FFS) was significantly better with adjuvant chemotherapy (HR 0.74, P = .03), although by year, the advantage was significant only at year 3 (see Table 2).
Dr. Strauss said that early stopping decisions in clinical trials are potentially problematic because early indications of treatment effectiveness may decline over time. He pointed out that, due to the small number of patients, this study wound up with only a 40% power to show a significant survival difference.
"Updated analysis no longer shows a significant overall survival advantage for adjuvant chemotherapy in stage IB NSCLC. CALGB 9633 can be interpreted as a negative study. However, the study did not have sufficient statistical power to detect smaller survival differences that are nonetheless clinically important," Dr. Strauss said.
He pointed out that the study did show significant advantages in median FFS, 3-year FFS, and 2- and 3-year overall survival, providing some evidence that adjuvant chemotherapy is effective. "This raises the possibility that adjuvant chemotherapy may delay recurrence, even if it does not enhance curability," he said, adding that, according to exploratory analysis, the benefit may be limited to patients with large tumors.
"The results of CALGB 9633 do not mandate adjuvant chemotherapy as the standard of care in all stage IB patients, but we believe the results support continued consideration of adjuvant paclitaxel and carboplatin in stage IB NSCLC, particularly among those with tumors 4.0 cm in diameter or larger," Dr. Strauss concluded. "We also believe that these results support inclusion of stage IB patients in ongoing and future randomized clinical trials comparing different adjuvant regimens in early-stage NSCLC."
Data from the LACE Study
Jean-Pierre Pignon, MD, PhD, reported data from the Lung Adjuvant Cisplatin Evaluation (LACE) study, a pooled analysis of five randomized clinical trials including 4,584 stage IA-III NSCLC patients (abstract 7008). The five trials all analyzed results of cisplatin-based chemotherapy in completely resected patients. The primary endpoint was overall survival; median follow-up was 5.1 years.
The absolute difference in survival benefit at 5 years was 5.3% in favor of chemotherapy. Post hoc analysis showed that cisplatin/vinorelbine was marginally better than other drug combinations. However, Dr. Pignon pointed out that the protocol dose of cisplatin was higher in patients treated with cisplatin/vinorelbine than for those treated with other combinations. "The interaction with the associated drug(s) may be confounded by the cisplatin dose," he said.
The chemotherapy benefit varied with stage. The hazard ratios for stages IA, IB, II, and III disease were 1.41, 0.93, 0.83, and 0.83, respectively. There was no interaction between chemotherapy and patient sex, age, planned radiotherapy, or planned total dose of cisplatin. "Chemotherapy may be detrimental for stage IA patients, but such patients were not given the potentially best combination of cisplatin/vinorelbine in most cases," he said.
Dr. Pignon concluded that cisplatin-based adjuvant chemotherapy improves OS and DFS rates in NSCLC. "Vinorelbine plus 320 to 400 mg/m
2
of cisplatin appears to be the most promising drug combination," he said. "Despite the large number of patients, multivariate analyses were not able to assess the respective role of the associated drug and cisplatin dose. Cisplatin-based chemotherapy is certainly effective for stages II and III." As to early-stage disease, "results of the updated NSCLC meta-analysis that will be available in 2007 will have enough power to reach a conclusion," he said.
Adjuvant CT in Elderly Patients
Investigators from the National Cancer Institute of Canada (NCIC) reported that adjuvant chemotherapy is both effective and tolerable for elderly patients with NSCLC and that such patients should not be denied adjuvant chemotherapy merely on account of age.
Carmella Pepe, MD, of Princess Margaret Hospital, Toronto, reported on behalf of the NCIC JBR.10 study investigators (abstract 7009). JBR.10 compared adjuvant cisplatin/vinorelbine vs observation. This retrospective study examined the effect of age on survival and chemotherapy delivery and toxicity in NSCLC patients aged less than 65 (n = 327) and more than 65 (n = 155).
Results showed that adjuvant chemotherapy significantly increased overall survival for patients aged more than 65 (66% vs 46% for observation, HR 0.61, P = .04), without significantly increasing toxicity. Mean dose intensities of vinorelbine and cisplatin were significantly higher in younger patients, compared with those over 65, and the older patients received significantly fewer doses of both agents. Fewer elderly patients completed treatment and more refused treatment, compared with the younger patients.
There were no significant differences in toxicities, G-CSF use, or hospitalization by age group, except for myalgias and mood alteration, which were more frequent in the younger patients.
Dr. Pepe concluded, "Chemotherapy in the adjuvant setting should not be withheld from elderly patients on the basis of age alone."