Anti-IGF-1R antibody plus chemo active in advanced non-small-cell lung cancer
An antibody to the insulin-like growth factor type 1 receptor (IGF-1R), when given with chemotherapy, is active as first-line therapy in advanced non-small-cell lung cancer, especially squamous type, finds the first trial to test an IGF inhibitor in lung cancer. Daniel D. Karp, MD, of M.D. Anderson Cancer Center, reported the trial results at ASCO 2008 (abstract 8015).
ABSTRACT: The large majority of patients with squamous histology had a response in this phase II trial of an anti-IGF-1R antibody.

CHICAGO-An antibody to the insulin-like growth factor type 1 receptor (IGF-1R), when given with chemotherapy, is active as first-line therapy in advanced non-small-cell lung cancer, especially squamous type, finds the first trial to test an IGF inhibitor in lung cancer. Daniel D. Karp, MD, of M.D. Anderson Cancer Center, reported the trial results at ASCO 2008 (abstract 8015).
In the randomized phase II trial among patients with locally advanced or metastatic NSCLC, 97 patients were assigned to a TCI arm-paclitaxel, carboplatin, and the investigational CP-751,871 antibody (Pfizer) in a higher or lower dose-and 53 patients were assigned to a TC arm (paclitaxel and carboplatin).
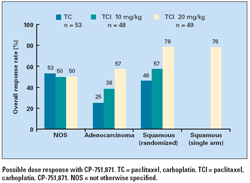
Some 46% of the patients had adenocarcinoma, 18% had squamous-cell tumors, 10% had large-cell tumors, and 26% had tumors types not otherwise specified (NOS).
Response rate
The overall response rate according to RECIST criteria was significantly higher with TCI than with

TC (54% vs 41%), Dr. Karp reported.
Further analyses suggested a dose response to the antibody component of TCI as well as a higher overall response rate with TCI among patients with squamous histology than among those with other tumor types (see Figure). The latter finding was supported by results in a single-arm post-trial extension study among 14 evaluable patients with squamous histology given TCI with higher-dose antibody.
“These responses were rapid and dramatic, and got our attention,” he commented of the shrinkage of bulky squamous tumors.
Among the randomized patients overall, median progression-free survival was 4.3 months for TC, 3.6 months for TCI with the lower-dose antibody, and 5.0 months for TCI with the higher-dose antibody. The hazard ratio for progression was 0.80 for higher-dose TCI, compared with TC (P = 0.07).
Among the subset of randomized patients with squamous histology, median progression-free survival was 4.3 months for TC and lower-dose TCI combined vs 5.6 months for higher-dose TCI.
Compared with their counterparts in the TC arm, patients in the TCI arm had higher rates of grade 3-4 neutropenia (30% vs 16%) and hyperglycemia (20% vs 8%).
“I think it’s intuitively clear that this agent should be associated with some extra hyperglycemia,” Dr. Karp said. Moreover, “we know these patients are getting steroids to get Taxol [paclitaxel], and we regularly see some elevations of blood sugar in our patients.” The hyperglycemia was quite manageable, he said.
“Our strategy for the future is to build on this with histology-specific studies,” Dr. Karp said. A trio of phase III trials are currently planned or initiated in patients with NSCLC.
One trial will compare TCI vs TC for untreated nonadenocarcinoma, a second will test the combination of erlotinib (Tarceva) and CP-751,871 against erlotinib alone in refractory nonadenocarcinoma, and the third will expand on the phase II trial among patients with all histologies excluding NOS, he said.