ASCO 2011: Large-Scale Study Shows Effectiveness of HPV Testing
One of the highlights of the released abstracts is “Cervical cancer risk for 330,000 women undergoing concurrent HPV testing and cervical cytology in routine clinical practice” (J Clin Oncol 29: 2011 (suppl; abstr 1508). The large-scale study showed the effectiveness of human papillomavirus (HPV) testing alone or in combination with cytology testing for identifying women at high-risk for cervical cancer development.
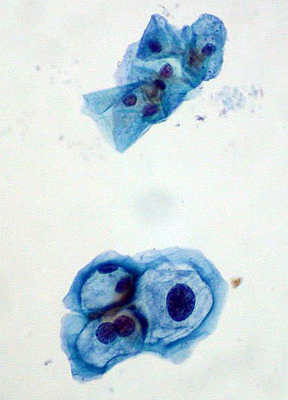
Normal squamous cells (top); HPV-infected cells with mild dysplasia (bottom)
One of the highlights from the annual meeting of the American Society of Clinical Oncology (ASCO) is a large-scale study that showed the effectiveness of human papillomavirus (HPV) testing alone or in combination with cytology testing for identifying women at high-risk for cervical cancer development.
The findings from the study, “Cervical cancer risk for 330,000 women undergoing concurrent HPV testing and cervical cytology in routine clinical practice” (J Clin Oncol 29: 2011 [suppl; abstr 1508]) will be presented by Hormuzd A. Katki, PhD at an oral abstract session on Monday June 6th.
The study sought to address whether concurrent HPV testing and cervical cytology is a better way to monitor cervical cancer risk compared to cytology testing alone in women 30 years old and over. The authors estimated cumulative 5-year incidence of cervical cancer and cervical intraepithelial neoplasia (CIN) grade III at Kaiser Permanente Northern California for 331,818 women that were enrolled in the co-testing program.
Overall, the study showed that a single HPV test was superior to a single Pap smear for predicting which women would develop CIN III or cancer within 5 years.
Cytology testing only modified the risks for women who were HPV-positive. The authors emphasize that these results show HPV-based screening to be a feasible practice to implement into routine clinical care.