ASCO 2011: Olaparib in Patients with Platinum-Sensitive Relapsed Serous Ovarian Cancer
A recent study demonstrated that the novel oral Poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib, provided a significant improvement in progression-free survival for women with serous ovarian cancer when used as a maintenance therapy.
A recent study demonstrated that the novel oral Poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib, provided a significant improvement in progression-free survival for women with serous ovarian cancer when used as a maintenance therapy.
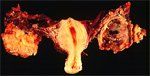
In this TAH-BSO specimen, the right ovary (on the left of the image) has been replaced by a solid serous carcinoma. The contralateral ovarian tumor is grossly cystic and could be termed a "cystadenocarcinoma." The patient had omental metastases and positive peritoneal fluid cytology. This cancer, which was discovered at exploratory laparotomy, apparently developed very rapidly; the patient had a normal pelvic ultrasound exam only 2 months before. Photograph by Ed Uthman.
The abstract, “Phase II randomized placebo-controlled study of olaparib (AZD2281) in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC)” (J Clin Oncol 29: 2011 [suppl; abstr 5003]) will be presented by Jonathan Ledermann, MD on Saturday June 4, 2011 at the Gynecologic Cancer Oral Abstract session at this year's annual meeting of the American Society of Clinical Oncology (ASCO).
The patients evaluated had all received at least two previous platinum chemotherapy regimens and had either a partial or complete response prior to starting the olaparib regimen. The progression-free survival was 8.4 months for patients taking olaparib compared to 4.8 months in the placebo group (P < 0.00001). The analysis of overall survival is still ongoing. Phase II trials with olaparib are also ongoing for breast and colorectal cancers.
The twice-daily treatment was reportedly well-tolerated and toxicities (mainly fatigue and anemia) were as previously reported with other studies.
Olaparib has previously shown activity in women with high-grade serous ovarian cancer with or without BRCA1 or BRCA2 mutations.