ASCO: Afatinib Improves PFS, Quality of Life in EGFR Mutation-Positive Lung Cancer
Patients with EGFR mutation-positive lung cancer had improved progression-free survival and better quality of life with new drug afatinib than with standard chemotherapy, according to results from a large phase III trial.
Patients with EGFR mutation-positive lung cancer had improved progression-free survival and better quality of life with the new drug afatinib than with standard chemotherapy, according to results from a large phase III trial.
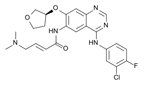
Chemical formula of afatinib
“This is a true global effort,” said James Chih-Hsin Yang, MD, PhD, of National Taiwan University Hospital, who led the study. The LUX-Lung 3 trial involved patients at 133 sites in 25 countries, testing if the irreversible ErbB family blocker afatinib could prolong survival among certain lung cancer patients. Unlike similar tyrosine kinase inhibitors, afatinib inhibits not only EGFR but other targets including HER2 (making it a breast cancer candidate drug as well).
Dr. Yang noted that EGFR mutations define a subgroup of patients with extreme sensitivity to this type of tyrosine kinase inhibitor. In LUX-Lung 3, 345 patients with stage IIIB/IV lung adenocarcinoma were randomized to either afatinib 40 mg/day (230 patients) or cisplatin 75 mg/m2 along with pemetrexed 500 mg/m2 (115 patients).
The primary endpoint of progression-free survival (PFS) showed a significant difference in favor of afatinib. The median PFS was 11.1 months for the study drug, compared with 6.9 months for the chemotherapy group; this yielded a hazard ratio for afatinib vs chemotherapy of 0.58 (95% CI, 0.43-0.78; P = 0.0004). Subgroup analyses showed advantages with afatinib for both men and women, for those with baseline ECOG scores of 0 or of 1, for patients who had never smoked, and other groups as well.
Notably, there was an even bigger improvement in PFS among patients with common genetic mutations. Patients with either the deletion 19 or L858R mutation had a median PFS of 13.6 months with afatinib, compared to 6.9 months for those patients randomized to cisplatin and pemetrexed.
Dr. Yang said that there was a near-universal occurrence of adverse events among patients in both arms of the trial. A total of 48.9% of afatinib patients and 47.7% of chemotherapy patients suffered drug-related adverse events of grade 3 or higher; 7.9% of afatinib and 11.7% of chemotherapy patients suffered drug-related events that led to discontinuation of the therapy.
In trials of advanced lung cancer, quality of life measures are especially important. Afatinib lengthened the time to deterioration of a number of lung cancer-related symptoms compared to cisplatin/pemetrexed; these symptoms included dyspnea, cough, and pain. The researchers measured quality of life using an EORTC questionnaire, and found significant improvements with afatinib for global health status, physical functioning, role functioning, and cognitive functioning, among other variables. There was a trend toward improved emotional functioning as well, but it did not reach significance.
Benjamin Solomon, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Australia, said that afatinib clearly provides benefit over other possibilities, but “the efficacy does come at a cost.” Dr. Solomon, who was not involved with the study, noted that more than 95% of patients who received afatinib experienced diarrhea, and 89% had rash/acne; these events in particular were much more rare among cisplatin/pemetrexed patients.
“I think the real questions for us are how this compares to the first generation EGFR inhibitors in this setting, and whether the potential increased efficacy outweighs any additional toxicity,” Dr. Solomon said. He noted that LUX-Lung 7 is now underway to answer that question, comparing afatinib to a first-generation EGFR inhibitor, gefitinib.