ASCO: Programmed Death 1 (PD-1) Inhibitor One of the “Most Exciting” New Melanoma Agents
The class of agents that target the Programmed Death 1 (PD-1) pathway was described at ASCO as “likely the most exciting new agents recently developed in melanoma.”
CHICAGO-Antoni Ribas, MD, of UCLA, in his discussion of the oral abstracts presented at the 2012 Annual Meeting of the American Society of Clinical Oncology on immune-stimulating antibodies for advanced melanoma, characterized the new class of agents that target the programmed death 1 (PD-1) pathway as “likely the most exciting new agents recently developed in melanoma.”
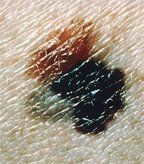
Superficial spreading melanoma arising from a dysplastic nevus. The 4-by-8 mm, pink-tan lesion with irregular borders at the upper left is a dysplastic nevus. Arising from it is an invasive malignant melanoma, with its characteristic blue-black color, notched border, and distorted surface. The gray area at the lower left represents tumor regression.
The programmed death-1 (PD-1) pathway has emerged as an important tumor-evasion mechanism. The mechanism involved, which shields tumor cells from destruction by the immune system, is similar to-yet distinct from-that of cytotoxic T lymphocyte antigen 4 (CTLA-4). The two principal components of the PD-1 pathway are PD-1, an inhibitory receptor expressed on the surface of activated T cells, and programmed death ligand-1 (PDL-1), which is expressed on cancer cells. When PD-1 and PDL-1 join together, the T cell’s ability to target the tumor cell is disarmed. Thus, targeting either PD-1 or PDL-1 can stimulate the immune system and enhance T cells’ ability to lyse tumor cells.
Data on a study assessing the safety and antitumor activity of BMS-936558, a fully human monoclonal antibody that blocks the PD-1 receptor on activated T lymphocytes, was presented by F. Stephen Hodi, Jr, MD, of the Dana-Farber Cancer Institute. The study included patients with a variety of cancers, including kidney cancer, non–small-cell lung cancer, and castration-resistant prostate cancer, as well as melanoma. Dr. Hodi focused on the 104 patients with advanced melanoma. The majority of these patients had received prior therapy, primarily immunotherapy. In the trial, patients received a variety of doses, up to 10 mg/kg; the maximum tolerated dose was not reached. Median duration of therapy was 15 weeks.
The key findings with regard to the safety of this new agent were that 20% of the patients on the trial had grade 3/4 adverse events, including two drug-related deaths (from pneumonitis); the most common of these were fatigue, diarrhea, nausea, and anemia. There was no relation between dose and incidence of adverse events.
The data on antitumor activity were encouraging-and clinical activity was seen at all dose levels. The overall response rate was 28%, with approximately two-thirds of responders exhibiting durable responses of more than 1 year. The progression-free survival rate at 24 weeks was 41%, and several patients had stable disease. Of note, several patients who were not categorized as responders because of the appearance of new lesions nonetheless had persistent reduction in their baseline target lesions.
Dr. Hodi noted that responses tended to fall into a few distinct categories, including some patients who had a substantial increase in lesions and others who demonstrated a rapid decrease. These patterns underscore the urgency of developing biomarkers. PDL-1 may be such a marker, but more study is needed to determine this.
Despite the similarity in the concepts behind blockade of CTLA-4 and blockade of the PD-1 pathway, Dr. Ribas noted that there are some noteworthy differences. Anti–CTLA-4 is less specific for antitumor T cells, while anti–PD-1 is more specific. Also, in a murine model, early death from autoimmunity was seen with anti–CTLA-4, whereas with anti–PD-1, autoimmunity had a late onset.
Despite the somewhat concerning fact that two patients on the trial had toxic deaths, Dr. Ribas concluded his discussion by hailing the class of PD-1/PDL-1 inhibitors as perhaps the “most impacting” new agents recently developed in melanoma, as well as in other cancers.