‘BloodBiopsy’ Test Detects Epithelial Cancer Cells
NEW ORLEANS-A test that detects epithelial cancer cells in circulating blood, and gives detailed information about their characteristics, was described in the late-breaking session of the 92nd Annual Meeting of the American Association for Cancer Research (AACR).
NEW ORLEANSA test that detects epithelial cancer cells in circulating blood, and gives detailed information about their characteristics, was described in the late-breaking session of the 92nd Annual Meeting of the American Association for Cancer Research (AACR).
The Circulating Cancer Cell Test (BloodBiopsy) addresses the need for early detection of cancer recurrence, early diagnosis of metastasis, and early information about the response to therapy, said principal investigator Paul O.P. Ts’o, PhD. "From 20 mL of blood, we can get a definitive indication that a patient has cancer and that the cancer is in the circulation. The consequences of this, however, we do not know yet," said Dr. Ts’o, professor of biochemistry, Johns Hopkins School of Hygiene and Public Health, and chairman and CEO of Cell Works Inc. (Baltimore), developer of the test.
"We can find one cancer cell in 1 to 2 mL of blood or one cancer cell in 10 to 12 million white blood cells. The approach we take is to exclude all the blood cells and leave only the cancer cells behind," he said at a press conference.
The procedure utilizes double-gradient sedimentation for the removal of most red blood cells and white blood cells as well as magnetic cell sorting for the additional removal of white blood cells. The epithelial cells that remain are intact, without the attachment of magnetic beads or other foreign material, as may be the case with positive-selection systems. The cells are fixed, stained with various specific molecular probes, and scanned with an automated spectroscopic system.
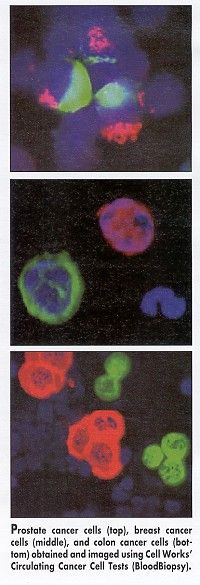
The spectroscope captures a fluorescent digital image at low magnification, and further analyses are performed at higher resolution. Cancer cells and markers are identified on the basis of intensity and blob analysis (see Figure).
This test can routinely provide five types of basic information about individual cancer cells found in the blood sample, Dr. Ts’o said.
1. The test shows the number of cancer cells in the sample. The recovery rate of epithelial cells is 50% to 80%. Preliminary data on blood samples from 13 prostate cancer patients indicate that if there are 3,000 circulating cancer cells in the whole body, several cancer cells would be detected in 20 mL of blood.
2. The test analyzes the nuclear DNA content of the epithelial cells found in blood relative to the patient’s white blood cells, reported as a DNA index. The epithelial cells have abnormally high DNA content compared to the normally diploid white blood cells on the same slide, and abnormally large two-dimensional nuclear area. These data confirm as neoplastic an observed epithelial cell with abnormal DNA content or nuclear size.
3. Levels of thymidylate synthetase and DNA fragmentation indicate growth status of the cell; such analysis is performed on the same slide to identify and compare the growing cells vs the dying ones.
4. The test reveals information indicative of chromosomal abnormalities.
5. The test shows the absence or presence of certain proteins, such as androgen and estrogen receptors. This provides information useful in designing therapeutic strategies and patient management, Dr. Ts’o said.
"Now we are studying the uses of this information. This method should be useful especially during the ‘silent period’ between treatment of the primary tumor by surgery and radiation therapy and a recurrence," he said, and clinical studies are currently pursuing this aim.
The test should also reveal much about the leakage of cancer cells from the primary and secondary tumors, as well as the fate of these cells in the body. Studies have strongly suggested that the circulating cancer cells can divide and multiply in the blood (Cancer 88:2787-2795, 2000)information previously unknown, Dr. Ts’o said. "These circulating microtumors can be the initial bridge for metastasis," he commented.
In discussing this report at the press conference, Carol Prives, PhD, of Columbia University, said that the study represents "a big advance" in early detection of occult cells. "The test takes advantage not only of well-known markers but also newer markers for changes in cancer cells," Dr. Prives said. "We know cancer cells will grow rapidly but at the same time will die rapidly. This test can assess DNA division as well as the DNA fragments that occur when cells start to die, so the technology is promising for being able to get microscopic dissections of circulating cancer cells in the blood."
Three Tests Available
Circulating Cancer Cell Tests are currently available from Cell Works for prostate, breast, and colon cancer, and the company is developing tests for other cancers, including gastric, liver, kidney, and lung cancer, and melanoma. The test is intended to be used on patients with known cancer etiology and is not a screening test. The company licenses the patented technology for the tests from Johns Hopkins University.
Cell Work’s state-licensed reference laboratory accepts samples from physicians in more than 40 states. For more information: www.cell-works.com.