Bone-Targeted Therapy in Early Breast Cancer
In this article, we describe the role of bone-targeted therapies, specifically for managing early breast cancer, by reviewing their bone-specific and cancer-specific benefits.
Oncology (Williston Park). 32(11):562-9.

Katarzyna J. Jerzak, MD, MSc, FRCPC

Jacques Raphael, MD, MSc, FRCPC

Danielle N. Desautels, MD, MSc, FRCPC

Phillip S. Blanchette, MD, MSc, FRCPC

Ivan Tyono, BScPhm, RPh

Kathleen I. Pritchard, MD, FRCPC

Table 1. Clinical Fracture Rates in Original Major Randomized Adjuvant AI Trials
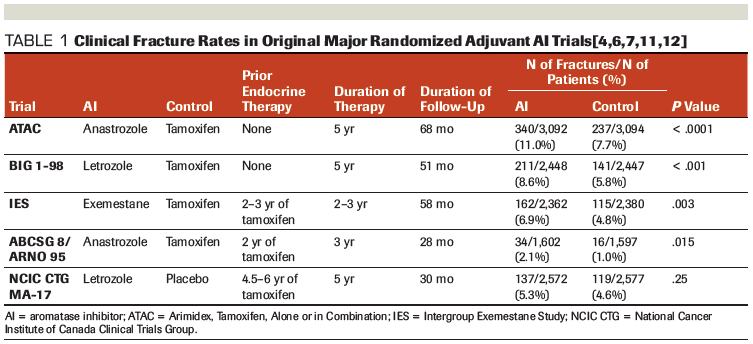
Table 2. Bisphosphonates Used Within the EBCTCG Meta-Analysis of Clinical Trials
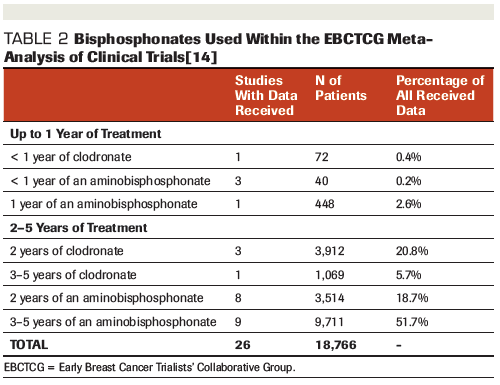
Table 3. Costs Associated With the Administration of Bone-Targeted Therapies for the Treatment of Patients With Early Breast Cancer
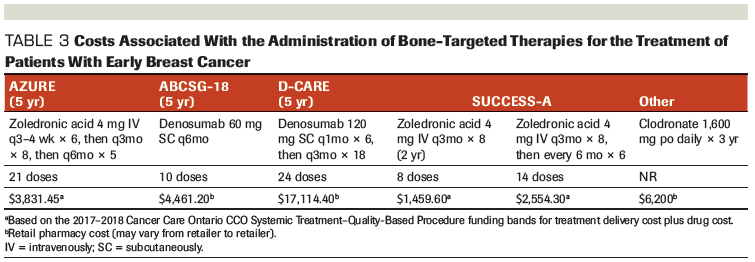
Table 4. Comparison of Denosumab Dosing and Patient Populations in the ABCSG-18 and D-CARE Studies
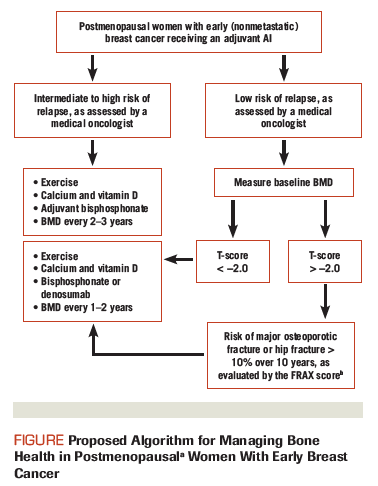
Figure. Proposed Algorithm for Managing Bone Health in Postmenopausal Women With Early Breast Cancer
Aromatase inhibitors (AIs) play an important role in the adjuvant treatment of hormone receptor–positive breast cancer, but they are associated with bone loss and increased fracture risk. Although several guidelines for the management of osteoporosis and osteopenia exist, their algorithms do not account for the use of AIs. In this article, we describe the role of bone-targeted therapies, specifically for managing early breast cancer, by reviewing their bone-specific and cancer-specific benefits.
Introduction
Aromatase inhibitors (AIs) play an important role in the adjuvant treatment of estrogen receptor–positive (ER+) breast cancer. By inhibiting the aromatase enzyme responsible for the peripheral conversion of androgens to estrogens, AIs effectively suppress circulating estrogen levels in women without residual ovarian function.[1] In the postmenopausal population, AIs significantly reduce breast cancer recurrence compared with tamoxifen.[2] As such, AIs are the preferred adjuvant endocrine therapy in postmenopausal women with early breast cancer. However, estrogen deprivation resulting from AI treatment is associated with bone loss and increased fracture risk. Because bone loss is generally asymptomatic until a fracture occurs, increased awareness of this issue is important to prevent progression to osteoporosis and resulting fractures.
Risk of Bone Loss and Fracture in Early Breast Cancer
A number of adjuvant trials on AIs in women with early breast cancer evaluated changes in bone mineral density (BMD) in subsets of the study populations. Among 108 evaluable women randomized to receive anastrozole or tamoxifen in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, those assigned to anastrozole had greater bone loss at the lumbar spine (LS) and total hip (TH) (LS, −6.08%; TH −7.24%) vs the tamoxifen group (LS, +2.77%; TH, +0.74%).[3] Separately, the Intergroup Exemestane Study (IES) evaluated 206 women who received 2 to 3 years of adjuvant tamoxifen for early breast cancer. Those who were randomized to switch to exemestane experienced a decline in BMD (LS, −2.7%; TH, −1.4%) after 6 months, while those who remained on tamoxifen demonstrated no change.[4] Over the following 18 months, BMD continued to decline in the exemestane group, albeit more slowly (additional −1.0% in LS and −0.8% in TH). Lastly, the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) MA.17 trial randomized 226 women to receive letrozole or placebo after 5 years of adjuvant therapy with tamoxifen. Women who received letrozole had significantly decreased BMD at 24 months (LS, −5.35%; TH, −3.6%) vs placebo (LS, −0.70%; TH, −0.71%).[5] In these studies, the use of AIs was also associated with increased biochemical markers of bone turnover.[3-5]
Fracture is arguably a more clinically relevant endpoint than bone turnover or asymptomatic decreases in BMD, and several of the original large adjuvant AI trials reported fracture data (Table 1). When evaluating these data, the increased fracture rate associated with AIs is greater in studies comparing an AI vs tamoxifen rather than placebo. In a large meta-analysis of individual data for 31,920 postmenopausal women with ER+ early breast cancer who were randomized to receive either an AI or tamoxifen, the 5-year fracture risk was 8.2% in the AI group vs 5.5% in the tamoxifen group (absolute excess, 2.7%; 95% CI, 1.7%–3.7%).[2] The increased fracture risk appears to be somewhat mitigated by prior tamoxifen use in those studies evaluating “switch” strategies.[4,6,7]
Evidence exists that the increased fracture risk associated with AIs decreases after stopping these medications. For example, the 10-year analysis of the ATAC trial found fractures were more frequent during active treatment with anastrozole vs tamoxifen (451 vs 351; odds ratio [OR], 1.33; 95% CI, 1.15–1.55; P < .0001), but not in the post-treatment follow-up period (110 vs 112; OR, 0.98; 95% CI, 0.74–1.30; P = .9).[8] With recent evidence supporting longer durations of adjuvant AI treatment in certain high-risk populations,[9] the implications for fracture risk must be considered. A recent publication-based meta-analysis on the toxicity of extended adjuvant therapy with AIs in early breast cancer found that longer treatment was associated with increased odds of fracture (OR, 1.34; 95% CI, 1.16–1.55; P < .001; number needed to harm, 72).[10]
Bone-Targeted Therapy Prevents Bone Loss, Fractures
Adding adjuvant bisphosphonates and receptor activator of nuclear factor kappa B ligand (RANKL) inhibitors to endocrine therapy in women with early breast cancer improves BMD from baseline, decreases bone loss risk, and reduces the risk of fracture thereafter. While both agents have shown success in these bone-specific endpoints, no “head to head,” comparisons are available; therefore, their data will be described separately herein.
Bisphosphonates
The risk of fracture in the original large randomized controlled trials on the efficacy of adjuvant AIs ranged from 2.0% to 11.0% (Table 1).[4,6,7,11,12] Bone health and fracture risk were also assessed in the more recent phase III randomized controlled trial AZURE, which evaluated whether adjuvant zoledronic acid reduces fractures in breast cancer patients.[13] This trial is the largest analysis on fracture risk during adjuvant bisphosphonate therapy among women with early breast cancer. A total of 3,360 women with stage II or III breast cancer were randomized to receive the standard of care alone or in combination with zoledronic acid. The zoledronic acid was administered at a dose of 4 mg via intravenous (IV) infusion for 5 years (every 3 to 4 weeks for 6 doses, then every 3 months for 8 doses, then every 6 months for the final 5 doses). After a median follow-up of 84.2 months, 8.3% of women sustained ≥ 1 fracture in the control arm, and 6.2% sustained a fracture in the zoledronic acid arm. The addition of zoledronic acid also increased time to first fracture (hazard ratio [HR], 0.69; 95% CI, 0.53–0.90; P = .0053), with most benefit occurring after a disease-free survival (DFS) event. This brings into question whether bisphosphonates might have the highest yield with respect to fracture prevention in the setting of recurrent disease.
The AZURE study was pooled with 26 additional trials in an Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis of 18,766 individual patients. Data regarding fracture risk were available for 13,341 women with early breast cancer. The risk of fracture was 6.3% among patients who received an adjuvant bisphosphonate vs 7.3% for those who did not (relative risk [RR], 0.85; 95% CI, 0.75–0.97; P = .02).[14] In addition, the 5-year fracture risk was reduced from 6.3% to 5.1%, with little effect in years 0 to 1 and most of the gain occurring in years 2 to 4. These results reflect various bisphosphonate regimens, including clodronate and aminobisphosphonates (Table 2). Although the relative efficacy of these regimens was not reported for the outcome of fracture incidence, the majority of individual patient data (97%) studied the use of adjuvant bisphosphonates for 2 to 5 years.
RANKL inhibitors
The ABCSG-18 clinical trial provides the most evidence to date on the use of denosumab in the adjuvant treatment of early breast cancer.[15] ABCSG-18 was a prospective, placebo-controlled, double-blind, multicenter, phase III clinical trial of 3,425 postmenopausal women with early-stage, hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer who underwent treatment with an AI between 2006 and 2013. Denosumab was administered at 60 mg subcutaneously (SC) every 6 months, with a median of 7 doses administered in the placebo and denosumab arms. Patients who received denosumab had a significant delay in time to first clinical fracture vs placebo (HR, 0.50; 95% CI, 0.39–0.65; P < .0001).[15] At 7 years following randomization, the clinical fracture rate in the denosumab arm was 11.1% (95% CI, 8.1%–14.1%) vs 26.2% in the placebo arm (95% CI, 15.6%–36.8%), and the benefits of therapy were seen across all subgroups. The fracture rate among patients in the placebo arm was estimated to be 10% at 3 years, 16% at 5 years, and 26% at 7 years.
Significant variations in the rates of fracture seen in ABCSG-18 vs the EBCTCG meta-analysis[14,15] can likely be explained by differences in patient characteristics, type and/or duration of therapy, clinical monitoring for fractures, and study design. Fracture events were not collected as a primary endpoint for the EBCTCG meta-analysis, so the incidence of fractures may be underestimated. Conversely, the ABCSG-18 trial-which had a primary endpoint of clinical fracture-may have reported less severe fractures not captured in other trials. This is due partly to regular x-ray monitoring for the presence of vertebral fractures in the ABCSG-18 trial. Further real-world, population-based evidence is needed to investigate the true burden of osteoporotic fractures in patients on endocrine therapy, as well as the potential benefit of adjuvant bone-targeted therapy or early osteoporosis intervention in this setting.
Fracture Risk Assessment
Evaluation methods to assess fracture risk are not in the scope of this article; however, some adjuvant trials evaluating the use of bone-targeted agents excluded women with low BMD, prior fracture, and those who previously received or are currently receiving these agents.[16] As recently reviewed by Black and Rosen in the New England Journal of Medicine,[16] organizations in Canada,[17] the United Kingdom,[18,19] and the United States[20] offer tools for the assessment of fracture risk, as well as guidelines for both pharmacologic and nonpharmacologic therapy. However, anticancer agents that accelerate bone loss are not included as risk factors in these algorithms, and methods to incorporate the use of AIs to quantitatively assess fracture risk are lacking.
Reducing the Risk of Breast Cancer Recurrence and Death With Bone-Targeted Therapy
Bisphosphonates
The benefit of bisphosphonates, including reducing the risk of breast cancer recurrence and death, varies in the literature. Older studies assessing the role of adjuvant oral clodronate in early breast cancer suggested a survival benefit, specifically among those who developed bone metastases.[21-23] However, a meta-analysis of three adjuvant clodronate trials did not confirm this survival benefit, possibly due to varying patient populations across the included studies and a lack of analyses by menopausal status.[24] Other studies examining the role of adjuvant zoledronic acid in pre- and postmenopausal women also showed varying results. For example, the AZURE trial found no association between the use of adjuvant zoledronic acid and either DFS or overall survival (OS).[13] However, in a prespecified subgroup analysis of postmenopausal women, a statistically significant survival benefit was noted.[13] In the ABCSG-12 trial of premenopausal women who received ovarian suppression with goserelin along with tamoxifen or anastrozole, an improvement in DFS (HR, 0.77; 95% CI, 0.60–0.99) was reported, but there was no OS benefit (HR, 0.66; 95% CI, 0.43–1.02).[25] This increases the possibility that any disease-related benefit of bisphosphonates may be limited to those women who are functionally postmenopausal. Indeed, preclinical evidence suggests that reproductive hormones may antagonize the efficacy of bisphosphonates against cancer cells in the bone.[26] Therefore, the effects of bisphosphonates are more pronounced in postmenopausal women with early breast cancer, in whom they can modify the microenvironment surrounding the cancer cells and decrease the risk of metastatic seeding.
Adam M. Brufsky, MD, PhDProfessor of Medicine and Associate Division Chief, Division of Hematology/Oncology, University of Pittsburgh School
of Medicine, Pittsburgh, PennsylvaniaMedical Director of the Magee-Women’s Cancer Program and Associate Director for Translational Investigation, UPMC Hillman Cancer Center; Co-Director of the Comprehensive Breast Cancer Center, University of Pittsburgh.What Have Prior Efforts to Improve Outcomes for Patients With Resectable Metastatic Colorectal Cancer Achieved?When we consider the use of oral bisphosphonates, intravenous bisphosphonates, or denosumab for early-stage breast cancer, we must ask ourselves two questions. First, do these agents prevent bone loss resulting from aromatase inhibitor (AI) use in a clinically meaningful manner? Second, do these agents prevent breast cancer recurrence in the bone?To answer the first question, osteopenia, osteoporosis, and bone fracture are clear and significant complications of AI use. When looking at all trials on adjuvant AI use, the degree of osteoporotic fracture stands out to me: it is in excess of 5% in some trials, and appears to increase the longer the AI is given. Both bisphosphonates (in any form) and denosumab have been shown to clearly maintain and improve bone mineral density in women on AIs, and denosumab clearly prevents fractures, even in women with normal bone density. Therefore, it is clear that a bone-targeted agent should possibly be considered for fracture prevention in postmenopausal women on an AI, and definitely in women at a higher risk of osteoporotic fracture.In answer to the second question, it is clear from many trials to date, as well as a meta-analysis, that bisphosphonates clearly prevent bone metastasis and improve breast cancer–specific survival and overall survival when given as adjuvant therapy to postmenopausal women. As long as 2 years of any bisphosphonate are given, absolute gains in the 10-year overall survival rate approach 3.3%. This comes close to reaching the gains seen from other interventions in this setting, which form the standard of care. Interestingly, denosumab did not improve 5-year disease-free survival in the D-CARE study, and the reason for the difference observed between bone-targeted agents in this setting is of intense interest. I agree with Jerzak et al that these agents should be reserved for women at higher risk of recurrence. Additionally, emerging new markers, such as lack of MAF amplification, may soon guide us as to which women, both pre- and postmenopausal, would benefit from a bone-targeted agent to prevent bone metastasis.Financial Disclosure:Dr. Brufsky serves as a consultant to Amgen, Novartis, and Sandoz.
To clarify whether adjuvant bisphosphonates have an effect on breast cancer–specific outcomes and OS, the EBCTCG meta-analysis included trials that utilized a variety of different bisphosphonates.[14] In postmenopausal women, highly significant reductions in any breast cancer recurrence (RR, 0.86; 95% CI, 0.78–0.94), distant recurrence (RR, 0.82; 95% CI, 0.74–0.92), bone recurrence (RR, 0.72; 95% CI, 0.60–0.86), and breast cancer mortality (RR, 0.82; 95% CI, 0.73–0.93 were observed. The absolute reduction in breast cancer recurrence to the bone was 2.2% (95% CI, 0.6%–3.8%), and the reduction in breast cancer–specific mortality was 3.3% (95% CI, 0.8%–5.7%). In this meta-analysis, the proportional reductions in bone recurrence and breast cancer mortality with bisphosphonate therapy did not depend on ER status or treatment with adjuvant chemotherapy. However, an interaction between menopausal status and treatment effect was observed, strengthening the evidence that bisphosphonates have different effects in pre- and postmenopausal women. It is notable that while bisphosphonates had no statistically significant benefit in pre- and perimenopausal women, they did not have significantly adverse outcomes, either. For this reason, the use of bisphosphonates for their bone-strengthening effects may still be reasonable in pre- and perimenopausal women when indicated.
RANKL inhibitors
The data on the use of adjuvant denosumab to improve breast cancer survival rates remain immature and uncertain. The ABCSG-18[15,27,28] and D-CARE[29] trials are the only studies to report breast cancer–specific outcomes with the adjuvant use of denosumab. The preliminary results of ABCSG-18, presented at the 2015 San Antonio Breast Cancer Symposium, showed a 7-year DFS benefit among women receiving denosumab (83.5%) vs placebo (80.4%), in addition to an increase in the primary endpoint of time to first clinical fracture (HR, 0.816; 95% CI, 0.66–1.00; P = .051).[27] Additional data were presented at the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting. In the intention-to-treat analysis of 3,425 postmenopausal women, the use of adjuvant denosumab was associated with improved DFS (HR, 0.82; 95% CI, 0.69–0.98; P = .025 by the logrank test) after a median follow-up of 72 months.[28] At 8 years, the DFS rate was 80.6% for denosumab vs 77.5% for placebo (absolute risk reduction, 3.2%). Given the significantly reduced risk of a first clinical fracture, patients were given the option in 2016 to unblind and cross over to the denosumab arm. This may have underestimated the true effect of denosumab on recurrence and survival outcomes.
In the D-CARE clinical trial, 4,509 women were randomized to receive either placebo or denosumab. Those randomized to denosumab received a more aggressive dose of 120 mg SC once a month for 6 months followed by 120 mg SC every 3 months for the next 4.5 years.[29] The HR for the primary endpoint of bone metastasis–free survival (BMFS) was 0.97 (95% CI, 0.82–1.14; P = .70 by the logrank test), with a similar effect seen in both pre- and postmenopausal women. Denosumab did not improve secondary endpoints, including DFS in the overall population, DFS in the postmenopausal subgroup, or OS. Among seven exploratory endpoints, only the time to bone metastasis as a first recurrence (P = .031) and time to first symptomatic bone metastasis (P = .024) improved with the use of denosumab.
The differing results in the ABCSG-18 and D-CARE studies is quite striking and may be related to the different patient populations. Notably, ABCSG-18 enrolled only postmenopausal women, whereas D-CARE included a mixed population of both pre- and postmenopausal women.[28,29] Patients enrolled in ABCSG-18 also had lower-risk breast cancers than those enrolled in D-CARE, with the majority having lymph node–negative disease (71.2% vs 5.8%) and the minority requiring neoadjuvant chemotherapy (24.7% vs 95.9%).[28,29] It is also notable that ABCSG-18 enrolled only women with hormone receptor–positive disease, most of whom had HER2-negative tumors (93.5%), whereas D-CARE included a broader representation of breast cancer molecular subtypes.[28,29] It is also possible that the ABCSG-18 results were more favorable when compared with D-CARE because less frequent and lower doses of denosumab are more effective among patients with early breast cancer.
Timing, Duration, and Choice of Bone-Targeted Therapy
Several trials have shown that the immediate initiation of bisphosphonate therapy is better than initiation at the time of BMD decrease, in terms of bone loss prevention.[30-33] The duration of treatment has varied across studies, from 1 year up to 5 years, but most of the data support the use of adjuvant bone-targeted therapy for 2 to 5 years. Further, with increasing use of extended adjuvant endocrine therapy, most clinical guidelines suggest a 3- to 5-year course of a bone-targeted agent.
Notably, in the SUCCESS-A trial, women with high-risk early breast cancer who were randomized to either a 5-year or 2-year duration of adjuvant zoledronic acid had similar OS, DFS, and bone recurrence rates at a median follow-up of 3 years.[34] The majority of women (> 70%) had hormone receptor–positive disease; 58% were postmenopausal, and all of them received adjuvant chemotherapy.[34] Therefore, the population didn’t optimally reflect patients who typically benefit from bisphosphonate therapy (ie, postmenopausal women with hormone receptor–positive breast cancer). Further, it is unclear how many patients received extended adjuvant endocrine therapy and whether bone-specific outcomes differed between the treatment groups. Regardless, longer follow-up will be required to increase confidence in these results.
To date, adjuvant bisphosphonates and denosumab have not been compared “head to head.” Among the trials within the EBCTCG meta-analysis, no significant heterogeneity was found between the effects of various bisphosphonate regimens on bone recurrence. Notably, similar benefits were found in patients who received the non-aminobisphosphonate clodronate (n = 5,053) vs those who received the aminobisphosphonates zoledronic acid or ibandronate (n = 9,290 and 3,072, respectively); however, no benefit was found in the smaller group of 953 women receiving pamidronate.[14] Unfortunately, the efficacy of these agents was not compared for other clinical outcomes in the EBCTCG meta-analysis.[14] Whether pamidronate truly has different effects on bone recurrence than other aminobisphosphonates is unclear based on this exploratory subgroup analysis. However, the increased efficacy of zoledronic acid is biologically plausible given that it is 100 times more potent than pamidronate.[35,36] Given that adherence to treatment may be improved with IV zoledronic acid vs oral bisphosphonates, with an associated cost savings,[37] the preferential use of zoledronic acid may be considered among women with early breast cancer (Table 3).
While denosumab has demonstrated excellent efficacy for fracture prevention, the differing breast cancer–specific outcomes seen in ABCSG-18 and D-CARE suggest that some caution should be used when considering its use in the adjuvant setting for early breast cancer. Although D-CARE enrolled a broader and higher-risk population than ABCSG-18 (Table 4), a lack of efficacy was still observed in postmenopausal, lymph node–negative, and hormone receptor–positive subgroups that was more akin to the ABCSG-18 population.[28,29]
Cancer Care Ontario’s 2016 Program in Evidence-Based Care, in collaboration with ASCO guidelines, recommends zoledronic acid at 4 mg IV every 6 months for 3 to 5 years or clodronate orally at 1,600 mg/day for 2 to 3 years for women in whom adjuvant bisphosphonate therapy is being considered.[38] However, these guidelines acknowledge that different durations of therapy may be employed.[34] Recommendations from other organizations, such as the St. Gallen International Expert Consensus guidelines[39] and the European Society for Medical Oncology[40] guidelines, were summarized by Hadji et al in 2017.[41]
As illustrated in the Figure, we propose that women with an intermediate-to-high clinical risk of breast cancer recurrence and/or those with an intermediate-to-high risk of fracture who are treated with an AI receive a bone-targeted agent.
Monitoring Bone Health
Few data exist on the ideal schedule for monitoring BMD in women on AIs. The supplemental 2013 National Comprehensive Cancer Network (NCCN) guidelines recommend dual energy x-ray absorptiometry (DXA) scans at least every other year[42]; the 2003 ASCO bone health update recommends yearly DXA screening for all postmenopausal women on AI therapy.[43] The Belgian Bone Club recommends that those with cancer treatment–induced bone loss and known osteopenia/osteoporosis undergo BMD monitoring every 1–2 years, and that those with a normal baseline bone density be monitored every 2–5 years.[44] All three guidelines are based on expert opinion.[42-44]
A more refined algorithm was proposed in 2008 by the UK Expert Group. All women on AIs with a T-score of < −1 are considered “high risk.” A bisphosphonate is recommended for all patients in this category. Even while on a bone-targeted agent, the group recommends that the BMD of women on bisphosphonate therapy be monitored every 24 months. “Medium risk” patients with a T-score > −1 are recommended to undergo BMD testing at a similar time interval.[45]
We propose a modified, more flexible approach for monitoring bone health in postmenopausal women on adjuvant AIs (Figure). First, we recognize that fracture risk is reflected not only by BMD results, but also by established risk factors, such as advanced age, low body mass index, fracture history, and steroid use, as assessed using the Fracture Risk Assessment Tool (FRAX). Hence, women at a low risk of breast cancer recurrence with a T-score > −2 may remain off bone-targeted agents with BMD monitoring every 1 to 2 years, depending on individual risk factors for fracture, rate of yearly bone loss, and preference based on the risks vs benefits of bisphosphonates.
In postmenopausal women on adjuvant AIs who already receive a bone-targeted agent, we recommend that BMD be monitored every 2 to 3 years; more frequent monitoring is unlikely to change management. This schedule may help identify women with declining BMD due to nonadherence, secondary causes of osteoporosis, and/or lack of efficacy of bone-targeted therapy. If reduced BMD is not adherence-related, referral to a bone health specialist is advised for evaluation of secondary causes of osteoporosis. If alternate causes are not found, switching the type of bone-targeted agent and/or endocrine therapy to tamoxifen (which strengthens the bone) may be considered.
We acknowledge that various methods for monitoring bone health in women taking adjuvant AIs are based on expert opinion, and FRAX score has not been validated in a breast cancer population.
Toxicity Considerations
Bisphosphonates and denosumab have a low risk of atypical fractures and osteonecrosis of the jaw (ONJ). The risk of ONJ in D-CARE was 5.4%; no confirmed cases of ONJ were reported in ABCSG-18.[28,29] A pooled risk of ONJ was not determined in EBCTCG, but a risk of up to 2% was reported.[14] Since bisphosphonates are cleared renally, renal function must be monitored to detect nephrotoxicity. Generally, women with a creatinine clearance < 30 mL/min should not receive bisphosphonates; denosumab may be utilized safely since it is not renally cleared. Calcium levels should be monitored before each treatment for both classes of bone-targeted therapy due to a risk of hypocalcemia.[34] Oral bisphosphonates may cause esophageal/gastric irritation or pain. Loss of vision or ocular pain due to inflammatory eye conditions may occur, and require prompt evaluation by an ophthalmologist.[34,46, 47]
Although data are limited, the toxicity of zoledronic acid and pamidronate appears to be similar.[48] The incidence of grade ≥ 3 events was comparable in multiple myeloma patients receiving zoledronic acid vs denosumab, with greater renal toxicity in the former group (17% vs 10%) and more hypocalcemia in the latter group (17% vs 12%).[49]
Conclusion
The literature supports adjuvant bisphosphonate use in AI-treated postmenopausal women with early breast cancer to prevent boss loss, reduce the risks of fracture and recurrence, and improve mortality. While denosumab shows strong benefit in reducing the risk of adjuvant endocrine therapy−associated fracture, data are insufficient to support adjuvant use to prevent bone metastasis and improve breast cancer survival. Studies on optimal dosing and duration of bone-targeted therapy are needed to optimize individual treatment.
Financial Disclosure: Dr. Jerzak served as a consultant and/or attended advisory boards for Genomic Health, Novartis, Purdue Pharma, and Roche. Dr. Pritchard served in consulting/advisory board roles for and received honoraria/travel support from AstraZeneca, Pfizer, Roche, Amgen, Genomic Health Inc., Novartis, and Eisai. Dr. Tyono served as a consultant to Teva. All other authors have no significant financial interest in or other relationship with the manufacturer of any product. or provider of any service mentioned in this article.
References:
1. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431-42.
2. Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341-52.
3. Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051-7.
4. Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119-27.
5. Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629-35.
6. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262-71.
7. Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455-62.
8. Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135-41.
9. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209-19.
10. Goldvaser H, Barnes TA, Seruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110.
11. Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60-2.
12. Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486-92.
13. Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997-1006.
14. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomized trials. Lancet. 2015;386:1353-61.
15. Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433-43.
16. Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254-62.
17. Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864-73.
18. Compston JE, Cooper AL, Cooper C, et al; National Osteoporosis Guideline Group. Osteoporosis: clinical guideline for prevention and treatment: executive summary. http://www.shef.ac.uk/NOGG/NOGG_Executive_Summary.pdf. Accessed October 12, 2018.
19. Compston J, Bowring C, Cooper A, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas. 2013;75:392-6.
20. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-81.
21. Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19:2007-11.
22. Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357-63.
23. Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006;8:R13.
24. Ha TC, Li H. Meta-analysis of clodronate and breast cancer survival. Br J Cancer. 2007;96:1796-801.
25. Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631-41.
26. Ottewell PD, Wang N, Meek J, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res. 2014;20:2922-32.
27. Gnant M. The impact of adjuvant denosumab on disease-free survival: results from 3,425 postmenopausal patients from the ABCSG-18 trial. Presented at the San Antonio Breast Cancer Symposium; December 2015; San Antonio, Texas:abstr S2-02.
28. Gnant M, Pfeiler G, Steger GG, et al. Adjuvant denosumab in early breast cancer: disease-free survival analysis of 3,425 postmenopausal patients in the ABCSG-18 trial. J Clin Oncol. 2018;36(suppl 15):abstr 500.
29. Coleman RE, Finkelstein D, Barrios CH, et al. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo-controlled D-CARE study. J Clin Oncol. 2018;36(suppl 15):abstr 501.
30. Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188-94.
31. Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192-201.
32. Llombart A, Frassoldati A, Paija O, et al. Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12:40-8.
33. Hines SL, Mincey B, Dentchev T, et al. Immediate versus delayed zoledronic acid for prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen-N03CC. Breast Cancer Res Treat. 2009;117:603-9.
34. Janni W, Friedl TWP, Fehm T, et al. Extended adjuvant bisphosphonate treatment over five years in early breast cancer does not improve disease-free and overall survival compared to two years of treatment. Cancer Res. 2018;78(suppl 4):abstr GS1-06.
35. Green RJ, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 420446, a new potent heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745-50.
36. Body JJ. Clinical research update: zoledronate. Cancer. 1997;80:1699-701.
37. Tyono I, Leung T, Boudreau A, et al. Business analysis of the transition from pamidronate to zoledronic acid in breast cancer and myeloma patients. Presented at the Canadian Association of Pharmacy in Oncology Annual Meeting; May 21-24, 2015; St. John’s, Newfoundland and Labrador, Canada.
38. Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:2062-81.
39. Goldhirsch A, Ingle JN, Gleber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol. 2009;20:1319-29.
40. Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22:2546-55.
41. Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1-12.
42. National Comprehensive Cancer Network. Breast cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 15, 2018.
43. Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042-57.
44. Body JJ, Bergmann P, Boonen S, et al. Management of cancer treatment-induced bone loss in early breast and prostate cancer-a consensus paper of the Belgian Bone Club. Osteoporos Int. 2007;18:1339-450.
45. Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(suppl 1):S3-S18.
46. Patel DV, Horne A, House M, et al. The incidence of acute anterior uveitis after intravenous zoledronate. Ophthalmology. 2013;120:773-6.
47. Etminan M, Forooghian F, Maberley D. Inflammatory ocular adverse events with the use of oral bisphosphonates: a retrospective cohort study. CMAJ. 2012;184:E431-E434. 48. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduced skeletal-related events in patients with osteolytic metastases. Cancer. 2011;91:1191-200.
49. Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomized, controlled, phase 3 study. Lancet Oncol. 2018;19:370-81.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.