In 2013, an estimated 9,290 cases of Hodgkin lymphoma (HL) were diagnosed in the United States, and 1,180 patients died from their disease. The median age at diagnosis is 38 years, although there is a bimodal age distribution with peaks around ages 20 and 65.[1] Mediastinal involvement is present in 60% of patients at diagnosis, and bulky mediastinal disease is seen in 20% to 25% of clinical stage I–II patients.[2] The definition of bulk has evolved as imaging modalities have changed. The most common one is based on results of a chest X-ray, and bulky disease (denoted by the letter “X”) is defined based on the ratio of the maximum width of the mediastinal mass and the maximum intrathoracic diameter on standing posterior-anterior X-ray (mediastinal mass ratio [MMR] > 0.33).[3,4] Other reports use a ratio with intrathoracic width at T5–T6.[5] Using CT, bulk is defined as a mass larger than 10 cm.[6] Studies suggest that chest X-ray findings correlate with the results of newer imaging modalities in approximately 90% of patients.[7]
Young women with stage I to II bulky mediastinal HL represent a unique population which poses specific challenges in diagnosis, treatment, and follow-up. We will focus on the management of patients with stage I–IIX mediastinal disease from age 18 to 40, as those younger than 18 years of age tend to be treated on pediatric protocols and are beyond the scope of this review.
Diagnosis and Staging
Patients with bulky mediastinal disease often present with superior vena cava syndrome, which makes management somewhat challenging, as expedited evaluation and treatment are required. In disease primarily localized to the mediastinum without involvement of peripheral nodes, a mediastinoscopy with biopsy may be required. Core needle biopsy or excisional biopsy is required due to the relative paucity of the pathologic Reed-Sternberg (RS) cells and extensive sclerosis seen in the most common subtype of classical HL (ie, the nodular sclerosing form). It is critical to obtain adequate tissue for appropriate immunohistochemical studies, as primary mediastinal non-Hodgkin lymphoma (NHL) also manifests in young patients with a similar clinical presentation, but management varies considerably. The RS cells of HL are nearly 100% positive for CD30 and 85% positive for CD15 by immunohistochemistry.[8] In contrast, primary mediastinal NHL cells are strongly positive for B cell–associated antigens including CD19, CD20, CD22, and CD79a.
Staging of bulky stage I–II HL is similar to that of advanced-stage disease, and requires laboratory testing and imaging as specified in the National Comprehensive Cancer Network (NCCN) guidelines.[9] In North America, bone marrow biopsy is usually performed in patients with B symptoms. Recent studies suggest correlation of positron emission tomography (PET) with bone marrow biopsy results, and challenge the role of bone marrow biopsy if no focal bone marrow involvement is seen on PET.[10] At Stanford, we perform a bone marrow biopsy in selected patients with B symptoms or cytopenias.
Based on the ability of PET scans to further characterize residual masses as a complete remission (CR) or a partial remission (PR), PET has emerged as a useful tool not only in staging but also in assessment of patient response at the end of therapy, especially when a residual mass is seen on CT.[11] Several studies have shown that the interim PET scan response during chemotherapy in advanced HL is prognostic.[12] Extrapolating from these results, risk-adapted strategies utilizing interim PET scans to tailor therapy in patients with stage I–IIX HL are ongoing, and described later in this article. These contemporary studies are designed with a central reading of PET results.[13] To limit inter-reader variability, the Deauville criteria were recently defined for the interpretation of interim or end-of-treatment PET scans using a five-point assessment score based on 18F-fluorodeoxyglucose (FDG) uptake in involved sites relative to the mediastinum. These criteria are being validated in several international trials using a risk-adapted therapy design.[13,14]
Prognosis
For early-stage disease in general, definitions of risk have been developed over several generations of clinical trials, but these were originally defined in the radiation therapy (RT) era. As previously stated, in North America patients with stage I–IIX disease are treated according to algorithms for advanced-stage HL.[15] The International Prognostic Score (IPS) is the most commonly used clinical prognostic index for advanced-stage disease,[16] and as the IPS does not include bulk as a risk factor, its utility in patients with stage I–IIX disease is limited. A retrospective study of 99 Swedish patients with stage IIB HL treated with 6 to 8 cycles of chemotherapy followed by 30–40 Gy involved-field radiation therapy (IFRT) demonstrated that bulky disease was the only statistically significant prognostic factor (P = .001) independent of the IPS.[17] In contrast, in Europe, the European Organisation for Research and Treatment of Cancer (EORTC) and the German Hodgkin Study Group (GHSG) stratify patients with bulk differently.[9] The EORTC considers all patients with bulky mediastinal involvement (MMR > 0.35) to have early-stage unfavorable (U) disease.[18] In the GHSG, patients with bulk (MMR > 0.33) and the additional presence of extranodal (EN) sites or B symptoms are considered to have advanced disease, whereas those without EN sites or B symptoms are classified as having early-stage U disease.[19] It is critical to appreciate these definitions by the various study groups to compare results across studies.
Treatment
Balancing efficacy with potential long-term toxicity poses a special challenge when treating a young woman with bulky mediastinal HL. Therapy has evolved over the past 2 decades due to recognition of late effects. Historical studies used extended-field RT (EFRT) and heavy alkylator-based therapy (mustard, Oncovin [vincristine], procarbazine, and prednisone [MOPP]), which resulted in increased morbidity and mortality due to a high risk of second cancers, especially breast cancer in young women, and cardiovascular disease.[20,21] Current treatment strategies use IFRT or involved-site radiation therapy (ISRT) combined with non-leukemogenic chemotherapy such as Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD).[22-25] Additionally, competing risks of therapy (such as the additive role of anthracyclines with RT in cardiovascular risk) need to be considered in making a treatment choice.[26] For the purposes of this review, we will restrict the discussion to contemporary clinical data from randomized phase III trials.
Combined modality therapy (CMT), consisting of chemotherapy followed by RT, is the standard of care for most patients with bulky mediastinal stage I–II disease. Developed by the Milan group, the most widely utilized chemotherapy is ABVD, based on a balance of efficacy and toxicity.[23] The GHSG HD8 trial was one of the largest trials to establish the efficacy of IFRT (as opposed to use of more extensive radiation fields) in the context of CMT. Mature results at 10 years confirmed the non-inferiority of IFRT in terms of freedom from treatment failure (FFTF), progression-free survival (PFS), and overall survival (OS) compared with EFRT, along with less acute toxicity and a lower incidence of secondary malignancies.[27] Currently, in North America, ABVD given for 6 cycles followed by IFRT is considered the standard of care for most patients with bulky stage I–II HL.[15]
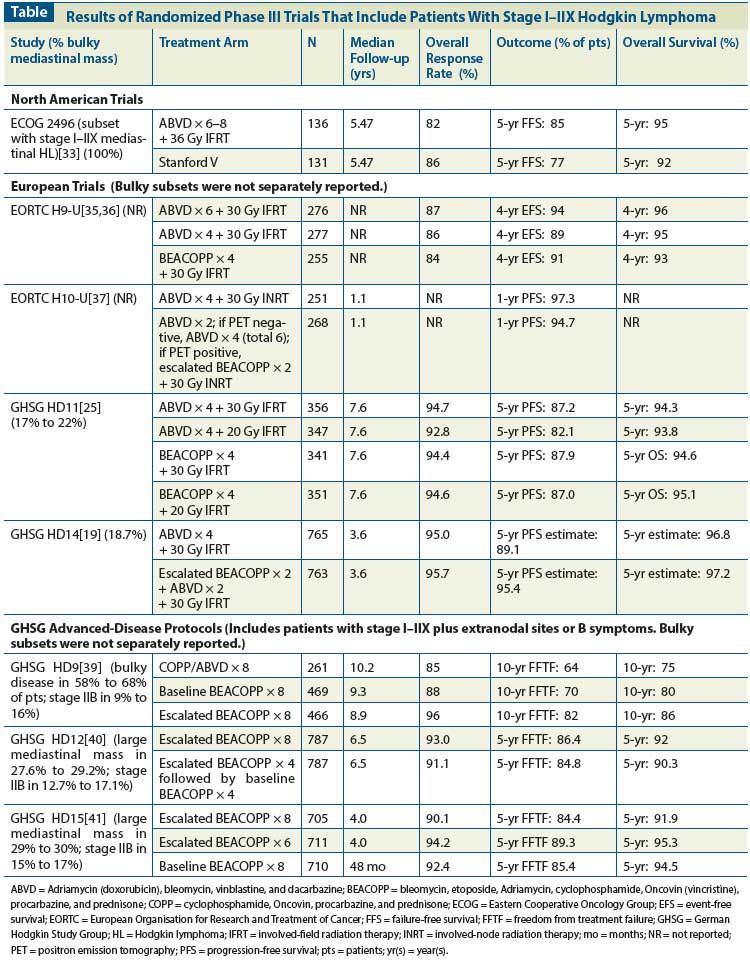
Other regimens have been developed with the goal of decreasing toxicity and maintaining high cure rates or even increasing efficacy, and randomized trials have now been completed. The Stanford V regimen (nitrogen mustard, doxorubicin, vinblastine, vincristine, bleomycin, etoposide, and prednisone) utilizes a CMT approach with weekly chemotherapy for 12 weeks followed by 36 Gy IFRT to sites that were ≥ 5 cm at the time of diagnosis. Compared with 6 cycles of ABVD, Stanford V exposes patients to a 50% lower dose of anthracycline (150 mg/m2 vs 300 mg/m2) and < 50% of the bleomycin dose (30 units/m2 vs 120 units/m2). Our single-institution study reported an OS of 96% and 5-year freedom from progression (FFP) of 89% in 142 patients with locally extensive and advanced HL.[28] These results were also confirmed by other studies, which used the protocol as published by Stanford.[29,30] Notably, the regimen preserves fertility and is not associated with an increased risk of secondary leukemia.[31,32] Recently, a randomized phase III United States Intergroup trial coordinated by the Eastern Cooperative Oncology Group (ECOG 2496) compared ABVD and Stanford V in patients with newly diagnosed stage III/IV HL or locally extensive disease (stage I–II) with bulky mediastinal adenopathy. For the subset of patients with locally extensive disease, RT was administered to both arms. In the ABVD arm, only the bulky mediastinal adenopathy was irradiated, whereas in Stanford V all sites ≥ 5 cm (including the mediastinum) were also irradiated. For the subset of patients with stage I–II bulky mediastinal disease (n = 267), the overall response rate (ORR) was high in both treatment groups (82% in ABVD arm vs 86% with Stanford V) with no differences for 5-year failure-free survival (FFS) (85% vs 77%, respectively; P = .13) and 5-year OS (95% vs 92%, respectively; P = .31) (Table).[33] Further, no differences in failure rate were seen after RT in the two arms (13% for ABVD vs 17% for Stanford V; P = .49) as well as no difference in patterns of failure, with < 10% in-field failures in both groups.[34] Importantly, the ECOG 2496 trial is the only study to date which has reported specifically on the subgroup of patients with stage I–IIX HL, and supports CMT as standard of care. It also provides a benchmark against which other studies need to be compared. Both regimens are reasonable choices in treatment of a young patient and are included in the NCCN guidelines.[9] Long-term follow-up is required to assess the effects of the reduced doses of anthracycline and bleomycin in Stanford V, compared with ABVD, on late cardiovascular and pulmonary risks.
In Europe, the BEACOPP regimen (bleomycin, etoposide, Adriamycin, cyclophosphamide, Oncovin, procarbazine, and prednisone), developed by the GHSG for patients with advanced disease, has also been evaluated in patients with stage I–IIX disease. In Europe, as discussed above, patients with bulky mediastinal stage I–II disease are variably defined. In the EORTC H9-U trial, 808 patients with U early-stage disease (including stage I–IIX disease irrespective of additional risk factors) were randomized to ABVD × 6 vs ABVD × 4 vs baseline-dose BEACOPP × 4, followed by 30 Gy IFRT in all arms. The 4-year event-free survival (EFS) rates were 94%, 89%, and 91% in the three arms, respectively (P = .23), and the 4-year OS rates 96%, 95% and 93% (P = .89). Chemotherapy-related toxicity was higher in the BEACOPP arm than in the ABVD arm. While data were not reported for the subset of patients with bulky mediastinal disease, the trial established ABVD × 4 plus 30 Gy IFRT as a standard arm for future comparisons (Table).[35,36]
The most recent trial conducted by the EORTC is the ongoing H10 trial, in which risk-adapted PET imaging was incorporated after ABVD × 2 for patients with favorable (F) or U newly diagnosed stage I–II HL.[37] For the U patients, the standard arm consisted of ABVD × 4 plus 30 Gy involved-node radiation therapy (INRT), while the experimental arm consisted of ABVD × 2 followed by PET. If the interim PET scan was negative, patients were treated with ABVD × 4 without RT (total ABVD × 6 cycles); if the PET was positive, patients were switched to escalated BEACOPP × 2 followed by 30 Gy INRT. At a planned interim analysis, with a median follow-up of 1.1 years, the experimental arms (without RT) for both the F and U subsets were closed early due to inferior PFS compared with the CMT arm. Specifically in the U early PET negative standard group (ABVD × 4 plus INRT), 2.8% of patients had events (progression, relapse, or death) compared with 6.0% in the U early PET negative experimental group (ABVD × 6).[37] The study has been amended, and currently RT is administered to all patients with early negative PET scan results. If the interim PET scan is positive, therapy is intensified to escalated BEACOPP followed by INRT. This part of the study is ongoing and no results are available to date.
In the GHSG, patients with U disease due to bulky mediastinal involvement are further classified as having limited or advanced-stage disease depending on the presence of additional risk factors. The HD11 trial for early U HL included early-stage patients with bulky mediastinal mass without extranodal sites or B symptoms who were randomized to four treatment arms: ABVD × 4 plus 30 Gy IFRT; ABVD × 4 plus 20 Gy IFRT; baseline BEACOPP × 4 plus 30 Gy IFRT; or baseline BEACOPP × 4 plus 20 Gy IFRT.[25] Patients with bulky mediastinal disease accounted for 17% to 22% of the overall study population. For the entire cohort of 1,395 patients, the FFTF at 5 years was 85%, OS was 94.5%, and PFS was 86.0% (Table). Baseline BEACOPP was more effective than ABVD when followed by 20 Gy IFRT (5-year FFTF difference, 5.7%), but no difference was seen when 30 Gy IFRT was used. The OS was similar in both arms, but World Health Organization (WHO) grade 3 or 4 toxicities were significantly higher with baseline BEACOPP vs ABVD (73.8% vs 51.5%, respectively, P < .001) and hospitalization more frequent (58.8% vs 42.6%, respectively). Notably, no subset analysis was specifically reported for the outcomes of patients with bulky mediastinal disease, and the overall conclusion of the authors was that the modified dose escalation using baseline BEACOPP did not significantly improve outcomes in early unfavorable HL. ABVD × 4 plus 30 Gy IFRT was recommended as a standard therapy for future comparison.
In the GHSG HD14 trial (with the same inclusion criteria as in HD11), patients were randomized to ABVD × 4 or escalated BEACOPP × 2 followed by ABVD × 2 (2+2). Both arms were followed by IFRT at 30 Gy. Overall 18.7% of patients had a large mediastinal mass. Although the primary endpoint of FFTF was superior in the intensified arm combining escalated BEACOPP and ABVD (a difference of 7.2% at 5 years; hazard ratio [HR] = 0.44; 95% confidence interval [CI], 0.30–0.66), no difference was seen in OS. WHO grade 3 or 4 acute toxicity was significantly more frequent in the BEACOPP arm (87.1% vs 50.7%).[19] Again, no separate data on patients who have bulky mediastinal disease have been presented to date in either the HD11 trial or the HD14 trial.
In contrast, patients with stage I–IIX HL with extranodal sites or B symptoms have been treated on protocols for advanced HL in the GHSG. Therapy has evolved from HD9 to HD12 and HD15 studies[38] (Table). None of these studies report on the outcomes of bulky stage I–II patients separately. The HD9 trial randomized unfavorable patients to cyclophosphamide, Oncovin, procarbazine, and prednisone (COPP) alternating with ABVD × 8; baseline BEACOPP × 8; or escalated BEACOPP × 8. RT was administered for initial bulky or residual tumors > 2.5 cm after chemotherapy. Stage IIB disease was found in 9% to 16% of patients. At 10-year follow-up, FFTF and OS were 64% and 75% in the COPP/ABVD arm, 70% and 80% in the baseline BEACOPP arm, and 82% and 86% in the escalated BEACOPP arm, respectively. Escalated BEACOPP led to significantly more acute hematologic toxicity (with leukopenia occurring in 71% of patients in the COPP/ABVD arm vs 98% in the group treated with escalated BEACOPP). Additionally, the incidence of secondary acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) was increased in the escalated BEACOPP arm (3.2% vs 0.4% with COPP/ABVD). Thus, the HD9 study established escalated BEACOPP as the treatment of choice for patients with selected unfavorable early-stage HL and advanced-stage disease in Germany.[39]
In an attempt to decrease the toxicity associated with 8 cycles of escalated BEACOPP, the HD12 trial evaluated four arms in patients with advanced-stage HL: escalated BEACOPP × 8 followed by RT for responding patients with initial bulk ≥ 5 cm or residual disease ≥ 1.5 cm; escalated BEACOPP × 8 without RT; escalated BEACOPP × 4 followed by baseline BEACOPP × 4 plus RT; and escalated BEACOPP × 4 followed by baseline BEACOPP × 4 without RT.[40] Again, as in the HD9 study, hematologic toxicity was greater in the escalated BEACOPP arms. The primary outcome of FFTF in the pooled chemotherapy arms was not significantly different (86.4% in the escalated BEACOPP arms vs 84.8%), nor was 5-year OS (92% in the escalated BEACOPP arms vs 90.3%), again supporting the use of escalated BEACOPP × 8 for selected early-stage unfavorable or advanced-stage patients.
The next GHSG study, the HD15 trial, utilized a risk-adapted RT strategy based on PET scan at the completion of chemotherapy. Patients were randomized to one of three arms in this large noninferiority trial: escalated BEACOPP × 8, escalated BEACOPP × 6, or baseline BEACOPP × 8. If a 2.5-cm mass or larger was noted on PET scan after completion of chemotherapy, patients received RT at a dose of 30 Gy. Best results were seen with escalated BEACOPP × 6 followed by PET-guided RT with a 5-year FFTF of 89.3 % and OS of 95.3 % (compared with an OS of 91.9% in the group receiving escalated BEACOPP × 8 and 94.5% in the baseline BEACOPP arm). Additionally, patients in the escalated BEACOPP × 8 arm had significantly more secondary cancers compared to the arm with escalated BEACOPP × 6 (4.7% in escalated BEACOPP × 8 and 2.4% in escalated BEACOPP × 6, P = .02).[41] The PET-adapted strategy resulted in a decline of RT use from 71% in the HD9 trial to 11% in the HD15 trial, and it established escalated BEACOPP × 6 +/− RT as a standard of care within the GHSG.[41]
Thus, various CMT approaches have been evaluated in randomized clinical trials. Although the efficacy of upfront escalated BEACOPP appears to be better than that of ABVD or Stanford V in terms of PFS, minimal improvement in OS and concerns regarding toxicity have limited the adoption of this regimen in North America. Universal sterility has been reported despite the use of gonadotropin-releasing hormone analogs with 6 to 8 cycles of escalated BEACOPP.[42] This particular side effect makes using escalated BEACOPP a less appealing therapeutic choice in a young woman. While fewer cycles of escalated BEACOPP (as utilized in HD14) are associated with lower sterility rates, no data are reported specifically on the subgroup of patients with bulky mediastinal disease. It will also be useful to compare outcomes of patients with bulky stage I–II disease treated on early-stage U vs advanced-stage protocols within the GHSG and EORTC, to evaluate whether outcomes differ. To our knowledge, such data are not available.
The risk of RT-related late effects raises the question of utilizing chemotherapy alone, especially in young females. However, there are no contemporary trials comparing chemotherapy alone to CMT. A study from Italy prospectively evaluated consolidation RT vs observation in patients with bulky HL with negative PET scans post chemotherapy with vinblastine, etoposide, bleomycin, epirubicin, and prednisone (VEBEP) × 6.[43] After VEBEP, 160 patients with bulky HL had PET-negative residual masses. They were divided into two well-matched groups to receive 32 Gy RT to areas of bulk (> 5 cm) or undergo observation. Relapses were significantly increased in the observation group after a median follow-up of 40 months (14% vs 4% in the CMT group, P = .03), and all relapses involved the bulky site and contiguous nodal regions, suggesting a false-negative rate of 14% for a post-chemotherapy PET scan in this series.
While it is tempting to omit RT in patients with a negative PET scan after chemotherapy, it is important to note that the only prospective data supporting this are based on the GHSG HD15 trial, in which the chemotherapy backbone was escalated BEACOPP for 6 cycles.[41] It is premature to extrapolate these results to potentially improved outcomes when using ABVD chemotherapy, and it is important to wait for results of the ongoing Cancer and Leukemia Group B (CALGB) trial 50801. In the latter trial, patients with stage I–IIX HL are treated with ABVD × 2 followed by interim PET. Patients with negative PET receive ABVD × 4 without RT (total ABVD × 6), while patients with positive interim PET switch to escalated BEACOPP × 4 followed by 30 Gy IFRT.
At Stanford, our institutional preference is to treat young female patients with bulky stage I–II mediastinal HL with the Stanford V regimen. We prefer this over ABVD × 6 followed by IFRT due to the significantly lower doses of Adriamycin and bleomycin used. For patients who cannot commit to a weekly chemotherapy regimen, we use ABVD for 4–6 cycles plus ISRT as outlined by the NCCN guidelines.[9] Patients treated with Stanford V are restaged with a PET scan at the completion of chemotherapy. ISRT to sites ≥ 5 cm and for residual PET-positive sites is administered within 2 to 3 weeks of completion of chemotherapy for patients with a Deauville score of 1–3 (30 Gy for Deauville 1–2, 36 Gy for Deauville 3).[14] Patients with residual PET-positive sites (Deauville 4) are re-biopsied prior to RT, as our institutional data suggest that PET positivity at the end of Stanford V was a significant predictor of FFP.[44]
Ongoing trials
Several ongoing trials evaluate the role of RT or dose escalation based on interim PET scanning results. In the ongoing ECOG 2410 trial, patients with stage I–IIX HL receive 2 cycles of ABVD followed by a PET scan. If the PET results show no evidence of metabolically active disease on central review, patients receive 4 more cycles of ABVD followed by 30 Gy INRT; if the PET scan shows residual disease, patients receive escalated BEACOPP × 4 followed by 30 Gy INRT. As mentioned, the ongoing CALGB 50801 trial uses similar response-based therapy for patients with newly diagnosed stage I–IIX HL but omits RT in patients with a negative interim PET scan. The ongoing GHSG HD17 trial evaluates intermediate-stage HL with the 2+2 regimen; patients in the standard arm receive IFRT and those in the experimental arm receive INRT only if PET results are positive post chemotherapy.
For patients who have advanced-stage disease, incorporation of the recently approved antibody-drug conjugate brentuximab vedotin is also being evaluated in the frontline setting. Currently for patients who have stage I–IIX disease with B symptoms or extranodal disease being treated as advanced-stage disease in the GHSG, the ongoing HD18 trial evaluates the role of interim PET scan after escalated BEACOPP × 2; patients who have a negative interim PET scan receive 2 further cycles of escalated BEACOPP, but those with a positive interim PET scan receive 6 further cycles of escalated BEACOPP with the addition of rituximab.
Specifics of radiation therapy
Management of Young Women With Bulky Mediastinal HL
- The standard of care for patients with bulky mediastinal HL is combined modality therapy. This management approach poses special challenges in young women, because of potential short-term considerations (eg, the need to make decisions about fertility preservation) and long-term effects of therapy (the risk of cardiac disease and second cancers).
- In North America, cooperative group protocols often include patients with bulky stage I–II mediastinal disease together with advanced-stage patients, whereas in Europe additional prognostic factors are used to further risk-stratify patients into an early-stage–unfavorable category. The US Intergroup study ECOG 2496 suggests that for this subset two effective strategies are ABVD for 6 cycles followed by 36 Gy mediastinal radiation therapy (RT) and the Stanford V regimen for 12 weeks followed by 36 Gy involved-field RT.
- Current trials utilize risk-adapted designs based on interim PET scan, refinements of RT (including
reduction of field size and the use of protons), and incorporation of highly effective targeted agents, such as brentuximab vedotin, in frontline regimens. - Ultimately it is critical to tailor therapy at an individual level in order to maintain high cure rates and minimize toxicity.
Consolidation RT after chemotherapy has been explored in a variety of recent trials using different risk-stratification algorithms. Studies consistently demonstrate a superior FFP by the addition of RT.[45] In some older studies, long-term OS was compromised by the addition of RT; this was largely attributable to an increased risk of cardiovascular disease and second malignancies for patients in whom higher doses and volumes of RT were employed.[46] While these results may be of historical interest, current RT techniques have evolved, with use of smaller fields (IFRT, ISRT, INRT) and lower radiation doses (20–30 Gy). Classic mantle irradiation (which included the bilateral axillae) led to a 2.7-fold increased risk of breast cancer compared with the risk to women who received mediastinal irradiation alone at similar doses. The risk for secondary breast cancer was even higher among patients treated before age 21, in whom the cumulative incidence of breast cancer 30 years after treatment was 26%.[46]
Recently RT has evolved even further, from the narrow radiation fields employed by IFRT to even greater volume reduction with the more focused fields used in INRT and ISRT.[22] Both INRT and ISRT are alternatives to IFRT that restrict the size of RT fields and minimize exposure of uninvolved organs. At Stanford, we have adopted the ISRT fields advocated by the International Lymphoma Radiation Oncology Group and the NCCN.[9,22] The regions to be treated are defined by the staging PET-CT scan. Initially involved nodes and extranodal sites (by PET or CT) are identified by fusing those images with images from the CT simulation scan. The nodal volume and extranodal extension is identified as the gross tumor volume (GTV). This is extended 1.5–2 cm cranially and caudally to identify the clinical target volume (CTV). An additional 0.5 cm is added to the CTV to define the planning target volume (PTV). Plans for both conventional 3-D conformal and intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) are evaluated with respect to doses to the organs at risk (OAR). The intent is to minimize the risk for radiation pneumonitis by limiting the mean lung dose to 15 Gy (after ABVD) or 17 Gy (after Stanford V). The mean heart dose is limited to 20 Gy. The V4 (volume exposed to a dose > 4 Gy) of the breasts is defined and kept as low as possible, since doses as low as 4 Gy have been associated with an increased risk for breast cancer.[47] Patients are treated with 1.5-Gy daily fractions to a total dose of 30–36 Gy.
Proton therapy may also have a role in the treatment of HL, with the goal of increasing the therapeutic ratio and decreasing the involvement of surrounding critical organs.[48] The depth-dose characteristics of protons are such that one can provide greater sparing of the heart, esophagus, and posterior mediastinal structures and at the same time minimize the “low-dose bath” irradiation effects on the lungs and breasts that are associated with IMRT and VMAT therapy. These contemporary RT techniques are being rapidly adopted and, it is hoped, will further reduce the RT-related risks of treatment.
Surveillance
With contemporary approaches, approximately 80% to 90% of patients with stage I–IIX disease will be cured. The guidelines for follow-up are outlined in the NCCN recommendations.[9] Of note, PET-CT is not recommended for routine surveillance in patients achieving a CR, owing to a high false-positive rate.
RT is a particular risk factor for development of breast cancer in young women. An annual screening mammogram is recommended 8 to 10 years after completion of RT or starting at age 40, whichever occurs first. Breast magnetic resonance imaging complements mammography for screening and is recommended by American Cancer Society guidelines for women who were exposed to chest irradiation before age 30.[49]
Although modern radiation techniques significantly decrease the risk of cardiovascular disease compared with mantle field RT, cardiotoxic chemotherapy, such as anthracyclines and vincristine, also contributes to cumulative cardiac risk.[26] Stress testing and echocardiogram have been suggested as part of surveillance in patients 10 years after completion of therapy.[9] More important is lifestyle modification to manage additional risk factors, such as smoking, obesity, hyperlipidemia, hypertension, and diabetes.[50]
As many patients are lost to follow-up by oncology practices after 5 years, it is important to provide patients with an end-of-treatment summary with documentation of potential risks. Every effort should be made to follow these survivors in dedicated clinics geared towards evaluation of long-term risks and toxicities.
Conclusion
Although the cure rate remains high in women who present with bulky mediastinal stage I–II HL, the challenge remains to balance efficacy and minimize long-term toxicities. The use of risk-adapted chemotherapy strategies with interim PET scanning, improved risk stratification at diagnosis, and emerging novel treatments may allow for high cure rates while minimizing exposure to toxicity from chemotherapy and RT. Important current clinical trials will help to further define optimal management for these patients.
Financial Disclosure:Drs. Advani and Hoppe are consultants to Clarient, and Dr. Advani receives research funding from Seattle Genetics. Dr. Percival has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30.
2. Hughes-Davies L, Tarbell NJ, Coleman CN, et al. Stage IA-IIB Hodgkin's disease: management and outcome of extensive thoracic involvement. Int J Radiat Oncol Biol Phys. 1997;39:361-9.
3. Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630-6.
4. Mauch P, Goodman R, Hellman S. The significance of mediastinal involvement in early stage Hodgkin’s disease. Cancer. 1978;42:1039-45.
5. Bonfante V, Santoro A, Viviani S, et al. Early stage Hodgkin’s disease: ten-year results of a non-randomised study with radiotherapy alone or combined with MOPP. Eur J Cancer. 1992;29A:24-9.
6. Bradley AJ, Carrington BM, Lawrance JA, et al. Assessment and significance of mediastinal bulk in Hodgkin’s disease: comparison between computed tomography and chest radiography. J Clin Oncol. 1999;17:2493-8.
7. Kriz J, Mueller RP, Mueller H, et al. Large mediastinal tumor mass as a prognostic factor in Hodgkin’s lymphoma. Is the definition on the basis of a chest radiograph in the era of CT obsolete? Strahlenther Onkol. 2012;188:1020-4.
8. von Wasielewski R, Mengel M, Fischer R, et al. Classical Hodgkin’s disease. Clinical impact of the immunophenotype. Am J Pathol. 1997;151:1123-30.
9. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Hodgkin lymphoma. 2013; version 2.2013.Available from: http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf.
10. El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30:4508-14.
11. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-86.
12. Gallamini A, Rigacci L, Merli F, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica. 2006;91:475-81.
13. Gallamini A Kostakoglu L. Interim FDG-PET in Hodgkin lymphoma: a compass for a safe navigation in clinical trials? Blood. 2012;120:4913-20.
14. Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824-33.
15. Connors JM. Positron emission tomography in the management of Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:317-22.
16. Hasenclever D Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506-14.
17. Glimelius I, Molin D, Amini RM, et al. Bulky disease is the most important prognostic factor in Hodgkin lymphoma stage IIB. Eur J Haematol. 2003;71:327-33.
18. Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin's lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol. 2006;24:3128-35.
19. von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol. 2012;30:907-13.
20. Alm El-Din MA, Hughes KS, Finkelstein DM, et al. Breast cancer after treatment of Hodgkin’s lymphoma: risk factors that really matter. Int J Radiat Oncol Biol Phys. 2009;73:69-74.
21. Longo DL, Glatstein E, Duffey PL, et al. Alternating MOPP and ABVD chemotherapy plus mantle-field radiation therapy in patients with massive mediastinal Hodgkin’s disease. J Clin Oncol. 1997;15:3338-46.
22. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2013 Jun 18. [Epub ahead of print]
23. Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease: long-term results. J Clin Oncol. 2004;22:2835-41.
24. Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:4634-42.
25. Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199-206.
26. Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:323-9.
27. Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601-8.
28. Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630-7.
29. Edwards-Bennett SM, Jacks LM, Moskowitz CH, et al. Stanford V program for locally extensive and advanced Hodgkin lymphoma: the Memorial Sloan-Kettering Cancer Center experience. Ann Oncol. 2010;21:574-81.
30. Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin’s lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390-6.
31. Horning SJ, Hoppe R, Advani R, et al. Efficacy and late effects of Stanford V chemotherapy and radiotherapy in untreated Hodgkin’s disease: mature data in early and advanced stage patients. Blood. 2004;104:Abstr 308.
32. Koontz MZ, Horning SJ, Balise R, et al. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol. 2013;31:592-8.
33. Advani R, Hong F, Fisher RI, et al. Randomized Phase III Trial comparing ABVD + radiotherapy and the Stanford V regimen in patients with stage I/II bulky mediastinal Hodgkin lymphoma: a subset analysis of the US Intergroup Trial E2496. Blood. 2010;116:Abstr 416.
34. Advani R, Hong F, Gordon LI, et al. Patterns of failure in patients with stage I/II bulky mediastinal Hodgkin lymphoma (HL) treated with ABVD + radiotherapy or the Stanford V regimen in the randomized phase III North American Intergroup Trial: E2496. Blood. 2011;118:Abstr 1603.
35. Thomas J, Ferme C, Noordijk E, et al. Results of the EORTC-GELA H9 randomized trials: the H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin’s lymphoma (HL). Haematologica. 2007;92:Abstr C010.
36. Ferme C, Divine M, Vranovsky A, et al. Four ABVD and involved-field radiotherapy in unfavorable supradiaphragmatic clinical stages (CS) I-II Hodgkin’s lymphoma (HL): preliminary results of the EORTC-GELA H9-U Trial. Blood. 2005;106:813.
37. Andre M, Reman O, Federico M, et al. Interim analysis of the randomized EORTC/LYSA/FIL Intergroup H10 trial on early PET-scan driven treatment adaptation in stage I/II Hodgkin lymphoma. Blood. 2012;120:549.
38. Diehl V, Behringer K. Could BEACOPP be the new standard for the treatment of advanced Hodgkin’s lymphoma (HL)? Cancer Invest. 2006;24:713-7.
39. Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548-54.
40. Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234-42.
41. Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791-9.
42. Behringer K, Wildt L, Mueller H, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010;21:2052-60.
43. Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48:1721-7.
44. Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol. 2007;25:3902-7.
45. Herbst C, Rehan FA, Skoetz N, et al. Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2011;CD007110.
46. De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239-46.
47. Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465-75.
48. Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012;83:260-7.
49. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
50. Ng A, Constine LS, Advani R, et al. ACR Appropriateness Criteria: follow-up of Hodgkin’s lymphoma. Curr Probl Cancer. 2010;34:211-27.