Cancer of the Cervix: Current Management and New Approaches
This article summarizes the current management of patients with newly diagnosed cervical cancer. The topics range from the management of early-stage disease to the phase III randomized studies that have established the current standard of care for patients with locally advanced cancer of the cervix. New approaches to combined-modality therapy with the goal of improving outcomes and decreasing complications are also described.
This article summarizes the current management of patients with newly diagnosed cervical cancer. The topics range from the management of early-stage disease to the phase III randomized studies that have established the current standard of care for patients with locally advanced cancer of the cervix. New approaches to combined-modality therapy with the goal of improving outcomes and decreasing complications are also described.
Worldwide, carcinoma of the cervix is the second most common cancer in women, with an incidence of approximately 500,000 new cases each year and an annual death rate approaching 200,000; many of these women present with advanced-stage disease.[1] Cervical cancer is less prevalent in the United States, but still accounted for over 10,000 new cases and 3,700 deaths in 2005 alone.[1]
Background
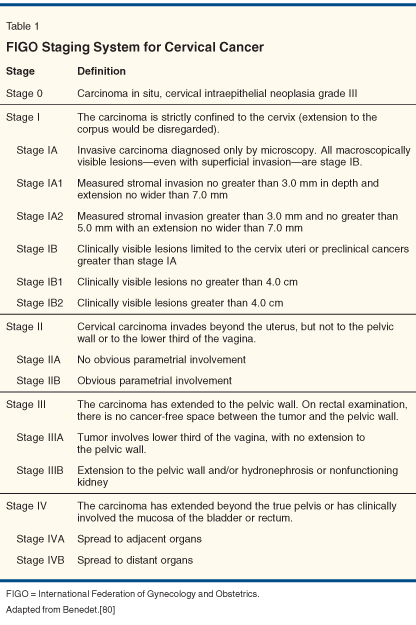
The management of patients diagnosed with cervical cancer is determined foremost by clinical stage at presentation, using the International Federation of Gynecology and Obstetrics (FIGO) staging system (Table 1). Surgery is commonly recommended for the definitive management of patients presenting with stage IA disease. The risk of recurrence following curative surgery for patients with stage IA disease is less than 1%.[2] The procedure consists of an extrafascial hysterectomy for stage IA1 disease or radical hysterectomy with pelvic lymph node dissection and possible para-aortic lymph node sampling for stage IA2 and higher. Patients with stage IA1 disease who are not surgical candidates or who desire to preserve fertility may be managed with a cone biopsy alone if negative margins can be obtained.
Prior to the 1990s, patients with stage IIB or higher advanced disease were treated with radiation alone. However, high rates of recurrence were noted both in the pelvis and distantly. In one series, nearly two-thirds of women treated with radiation alone had recurrent disease in the pelvis; these treatment failures were associated with higher rates of distant metastasis.[3]
Investigators attempted to improve upon these results with the addition of chemotherapy. Early studies examined the role of neoadjuvant chemotherapy followed by radiation. Unfortunately this treatment did not translate into an improvement in cure rates. In fact, one study even showed decreased overall survival.[4-10] Preliminary studies of concurrent chemoradiation (chemotherapy and radiation given simultaneously) demonstrated more promise and led to several US phase III trials (Table 2).[11-15] These studies demonstrated between a 30% and 50% survival benefit for concurrent cisplatin-based chemoradiation in locally advanced cervical cancer.
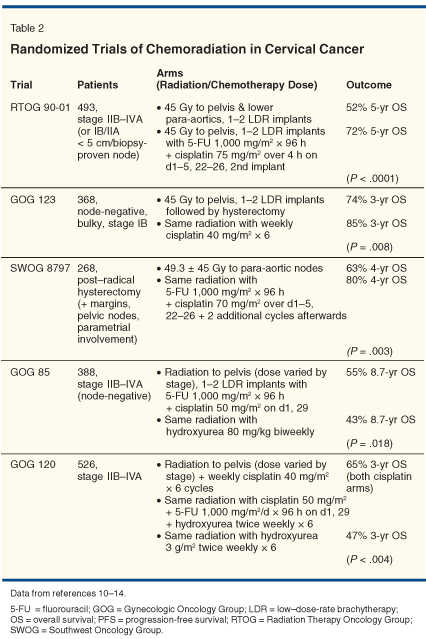
It should be noted that a single study from Canada (not listed in Table 2) failed to document a benefit of chemoradiation compared to radiation alone.[16] However, radiotherapy in this study was administered over a shorter period of time (median: 51 days) compared to other studies (62 to 64 days) and the study was not statistically powered to detect survival differences of less than 15%, a weakness noted by the authors.
Based on the results of these studies, the National Cancer Institute issued a clinical announcement in 1999 urging that strong consideration be given to treating patients diagnosed with invasive cervical cancer with concurrent platinum-based chemoradiation.[17] Despite this clinical announcement, the optimal management of patients diagnosed with stage IB1/IIA cervical cancer is still controversial. These patients may be managed with definitive surgery, definitive chemoradiation, or a combination of surgery and radiation with or without chemotherapy. Patients presenting at these stages are typically managed based on individual factors (eg, comorbidities, age, patient preference) and institutional expertise.
No randomized trial in the United States has compared definitive radiation therapy to radical hysterectomy for patients with stage IB/IIA cervical cancer. In Italy, Landoni and colleagues performed a study to address this question. Patients were randomized to either radical hysterectomy or radiation therapy, and there were no differences in overall survival. However, patients randomized to the surgery arm received adjuvant radiation if final pathology demonstrated extensive disease.[18] Severe complications, defined as morbidities requiring medical or surgical management, were significantly higher in the surgery group (28% vs 12%), and even more so in the patients who received both surgery and radiation. This study has also been criticized for the high rates of complications seen with surgery alone.
Radiotherapy Techniques
The standard of care in the US with regard to radiation techniques has been articulated in several texts as well as in the recommendations developed by the American Brachytherapy Society (ABS).[19] In patients treated with radiation therapy, the recommended total treatment duration is less than 8 weeks. Extending treatment beyond 8 weeks can reduce local control and has been estimated to reduce survival by approximately 1% per day.[20-22]
Low-Dose-Rate Brachytherapy
Brachytherapy is an integral part of the management of cervical cancer. It greatly enhances the curative potential of radiation therapy by providing the finest means of escalating the dose to the tumor while minimizing the dose to normal tissues.[23,24] Traditionally, intracavitary brachytherapy has been administered using low-dose-rate (LDR) radioactive sources, which requires hospitalization. Cesium-137 is the most commonly used radioactive isotope for LDR brachytherapy. Radiation exposure to hospital personnel has decreased considerably in recent decades with the use of afterloading techniques. Patterns of Care studies, which examine practice patterns in the United States, have shown that recurrences and complications are decreased when brachytherapy is used in addition to external-beam radiotherapy (EBRT).[25-30] Interstitial brachytherapy is generally utilized when a patient's disease cannot be optimally encompassed by intracavitary brachytherapy. Examples of such situations include extensive parametrial involvement, bulky primary disease, inability to insert tandem into endocervical canal, narrow vagina, or extensive vaginal involvement.
Two LDR insertions are generally recommended in most patients (especially those with larger tumors). By dividing brachytherapy into two insertions time for progressive tumor reduction is allowed, thereby improving dosimetric coverage of tumor with the second application. Dose specification is typically performed according to the classical or revised Manchester system.[31,32] Because the amount of radiation absorbed by the patient will vary with the distance from the radioactive sources, a particular point in the patient (point A) is often chosen as the prescription point. Point A is specified at 2 cm superior and 2 cm lateral from the cervical os. This point falls superior to the colpostats and runs parallel to the tandem, making it a more consistent specification point between patients.
The total dose to point A with EBRT and LDR brachytherapy should be approximately 85 Gy for locally advanced disease. This typically translates into 45 Gy EBRT and two LDR insertions of 20 Gy each. Delivery of these doses is predicated on the ability to keep the dose to normal tissues within acceptable limits.
High-Dose-Rate Brachytherapy
More recently, techniques using high-dose-rate (HDR) brachytherapy have been developed. As the name implies, radiation is given at a faster rate (200 cGy/min). This allows brachytherapy to be administered on an outpatient basis without the need for hospitalization. The primary disadvantage of HDR brachytherapy is the increased potential for late toxicity due to administration of larger doses per fraction.[33] Several studies comparing LDR to HDR brachytherapy have demonstrated comparable local control and survival.[34-46] Morbidity has been comparable between the two, with some studies demonstrating less rectal morbidity with HDR.
The ABS recommends HDR brachytherapy be interdigitated with EBRT. Because multiple fractions are required, waiting until the completion of EBRT to begin HDR could prolong treatment beyond 8 weeks. A variety of fractionation schemes have been used with HDR brachytherapy, depending on the dose of EBRT used. Doses used for HDR are lower than LDR because of differences in radiobiologic effects between the two. Suggested schedules range from 4 to 8 fractions at 5.3 to 7.5 Gy/fraction (based on EBRT doses ranging from 20 to 45 Gy). High-dose-rate fraction sizes of less than 7.5 Gy are recommended due to concerns of higher toxicity with larger doses per fraction.
Advances in Imaging
Positron-Emission Tomography
Prior to the availability of modern imaging, the evaluation for treatment of cervical cancer was limited to routine blood work, chest x-ray, intravenous pyelogram, and a barium enema. The FIGO system continues to allow only these tests to be used in clinical staging. Before positron-emission tomography (PET) imaging, our group and others utilized lymphangiography and cross-sectional imaging as a nonsurgical means to detect para-aortic and pelvic lymphadenopathy.[47] One meta-analysis concluded there were no significant differences between computed tomography (CT), magnetic resonance imaging (MRI), and lymphangiogram.[48] However, in one Gynecologic Oncology Group protocol, the sensitivity of CT in the detection of para-aortic lymph node metastasis was limited-only 34%.[49]
More recently, the use of PET imaging has been shown to improve detection of pelvic and para-aortic lymph node disease. Rose and colleagues from Case Western investigated a group of 32 patients who underwent PET imaging prior to surgical staging lymphadenectomy.[50] Most of these patients were FIGO stage IIIB. Positron-emission tomography had a sensitivity of 75% and specificity of 92% in predicting para-aortic nodal metastasis. In the pelvis, PET had a sensitivity and specificity of 100%. Reinhardt compared PET with MRI for lymph node detection in 35 patients with stage IB/IIA cervical cancer.[51] In that study, the sensitivity/specificity of PET was 91%/100%, compared to 73%/83% for MRI.
Positive nodes detected by PET imaging have been associated with poor long-term outcomes. Grigsby reported 2-year progression-free survival results in a cohort of 101 women who underwent CT and PET imaging prior to chemoradiation.[52] At a median follow-up of 15.4 months, 2-year progression-free survival was 64% in those with negative para-aortic nodes by PET vs less than 20% in those that were node-positive by PET. Positron-emission tomography has also been shown to correlate with tumor response during treatment.[53] It is also useful in the differentiation between persistent/recurrent disease and fibrosis after therapy.[54] At our institution, PET imaging is performed on all cervical cancer patients who are not receiving definitive surgery.
Image-Guided Brachytherapy
As discussed above, traditional doses for brachytherapy are based on a prescription to point A. A valid criticism of this approach is that it does not reflect the dose that the tumor actually receives. With the integration of various imaging modalities (ie, CT, MRI, PET) into the treatment process for external-beam radiation treatment planning, investigators have attempted to incorporate these modalities into brachytherapy treatment planning as well; the aim of this approach is to improve the efficacy and decrease the morbidity of such treatment.
Transrectal ultrasound has been identified as a means of obtaining real-time visualization of the target volume and normal tissues during interstitial needle placement, allowing for more accurate needle placement.[55] Use of CT has been shown to be helpful in identifying normal tissues such as bladder and sigmoid colon as well as determining uterine wall thickness.[56] Unfortunately, CT is not useful in identifying a tumor volume from surrounding structures (ie, bladder, uterus, rectum, vagina).
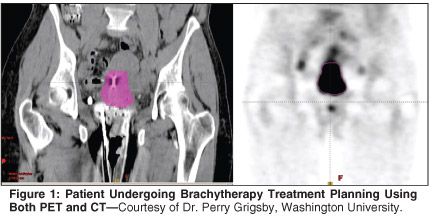
More recently, MRI and PET have shown promise in defining tumor volumes for brachytherapy planning. The use of PET in brachytherapy treatment planning has been pioneered by investigators at Washington University.[57] The hypothesis behind this approach is that functional imaging provides a distinct advantage over anatomic imaging in delineating tumor from critical structures (Figure 1). A significant advantage of this method is that standard applicators can be used with PET. This approach has been shown to be feasible without considerably increasing the time spent in treatment planning.
Compared to CT, MRI can more accurately delineate parametrial and vaginal tumor infiltration.[58] The issue of compatibility with traditional stainless steel applicators has been overcome with the introduction of MR-compatible applicators. The integration of MRI evolved primarily in Europe, in particular at the Institut Gustave Roussy and the University of Vienna.[59] Over time, this approach has allowed advancement from the practice of prescribing the dose to point A to prescribing the dose to cover a clinical target volume while sparing critical structures (bladder and rectum). While doses to the bladder and rectum have traditionally been reported using dose at a single reference point, MRI allows the doses to critical structures to be examined (and optimized) in a three-dimensional dose/volume relationship.
A multinational working group from Europe has also developed recommendations for defining tumor volumes using MRI.[60] One novel concept introduced in these recommendations is that of an intermediate-dose clinical target volume (ID-CTV), based on the extent of tumor noted on imaging at the time of diagnosis, and a high-dose clinical target volume (HD-CTV), based on extent of tumor following EBRT, at the time of brachytherapy. Two different dose prescriptions are applied to each of these volumes: a lower dose to the ID-CTV to eradicate any residual microscopic disease and a higher dose to the HD-CTV to eradicate all macroscopic residual tumor.
Recently, recommendations for specific dose/volume parameters to target volumes and critical structures from the working group have also been published.[61] Because of the superior soft-tissue resolution of MRI, the Imaged-Guided Brachytherapy Working Group has endorsed T2-weighted MRI for treatment planning.[58] A recent joint European and North American conference was held in Washington, DC, to help train North American physicists and radiation oncologists in the specifics of MRI-based target volume definitions (W. Small, personal communication). Further investigation of these modalities is necessary to ascertain if improved accuracy in treatment planning will translate into improved control rates and lower complications.
Improving Tumor Control and Decreasing Complications
Hyperthermia
During the mid-1990s, investigators in Europe hypothesized that improvements in the therapeutic ratio might be obtained with the addition of hyperthermia to radiotherapy. Hyperthermia involves heating tissues to temperatures of 40°C to 45°C, which preferentially destroys nutrient-deficient tumor cells in a low-pH environment.[62] These nutrient-deficient tumor cells also tend to be more radioresistant. The synergism between hyperthermia and radiation appears to be in blocking the repair of radiation-induced lesions.
One of the first randomized studies of hyperthermia with radiation was conducted by van der Zee and colleagues in the Netherlands.[63] They studied 358 patients with locally advanced pelvic tumors, including 114 patients with stage IIB-IVA cervical cancer. Hyperthermia was given once weekly, 1 to 4 hours after radiation, for a total of five treatments. The goal for each session was to heat the tumor to 42°C for 60 minutes. The complete response rate for patients with cervical cancer was 83% with hyperthermia vs 57% without (P = .003). Local control (61% vs 41%) and 3-year overall survival (51% vs 27%, P = .009) also favored the hyperthermia group. Toxicity rates were similar in both arms. Of note, the results with radiation alone were substantially worse than reported in other series. The authors believe their study population included patients with a worse prognosis, namely, younger patients with larger, node-positive tumors.
In contrast, a second multinational randomized study found no benefit for the addition of hyperthermia to radiation, along with increased toxicity.[64] However, this trial has been criticized for lack of quality control, insufficient statistical power, and substandard radiotherapy.
Given the standard of chemoradiation in the United States, investigators have sought to determine whether hyperthermia can produce additional benefit when concurrent chemoradiation is used. Results of a multinational phase I/II collaboration between the US, Norway, and the Netherlands suggests that "triple modality" is in fact both feasible and effective.[65] Complete response rate was 90% and 2-year overall survival was 78.5%. A phase III randomized trial has been launched to compare chemoradiation with or without hyperthermia; the target for accrual is 400 patients (W. Small, personal communication).
Intensity-Modulated Radiation Therapy
Intensity-modulated radiation therapy (IMRT) uses complex treatment-planning techniques to optimize radiation delivery to irregularly shaped volumes. Multileaf collimators available on many treatment machines allow for dynamic radiation delivery. The actual set of treatment beams and intensities is obtained using computer algorithms. Several institutions have published dosimetric studies with IMRT demonstrating decreased radiation to normal tissues without compromising dose to target volumes.[66-70]
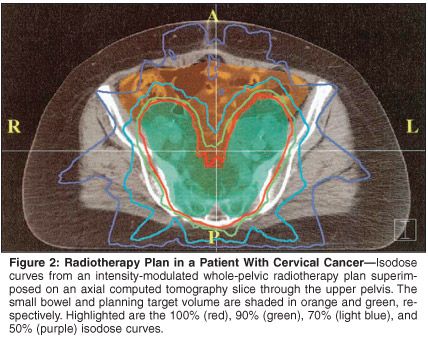
Mundt and colleagues at the University of Chicago reported their initial experience in 40 patients with gynecologic malignancies (over 60% had cervical cancer) treated with IMRT.[71] Compared to a cohort of 35 patients treated during the same time, patients treated with IMRT had significantly fewer gastrointestinal complaints (grade 2: 60% vs 91%, P = .002) and a trend toward fewer genitourinary complaints (grade 2: 10% vs 20%, P = .22) during radiation (Figure 2). To our knowledge, no long-term data on tumor control with IMRT have been published to date.
The Radiation Therapy Oncology Group (RTOG) is currently developing a protocol (RTOG 0418) to investigate the use of IMRT in posthysterectomy patients (for cervical or endometrial cancer [W. Small, personal communication]). Given the increased risk of gastrointestinal toxicity from radiation in posthysterectomy patients, this is a logical group in whom to study this potential means of reducing toxicity.
Amifostine
For decades, thiol-containing compounds such as amifostine (Ethyol) have been known to have radioprotective and chemoprotective properties.[72-74] However, few studies have explored its use in decreasing toxicity from the treatment of cervical cancer. Wadler and colleagues conducted a phase I trial with pelvic radiation, brachytherapy, and concurrent cisplatin with intravenous amifostine.[75] External-beam radiation therapy to the pelvis was given to a total dose of 39.6 Gy followed by brachytherapy to a total point A dose of 80 Gy, and cisplatin was delivered days 1-5 and 22-26 at 20 mg/m2 and additionally at 100 mg/m2 after brachytherapy; amifostine was delivered before each cisplatin treatment. With a dose-limiting toxicity of hypotension, the authors concluded a daily dose (during cisplatin infusion) of 825 mg/m2 was appropriate.
Gallardo evaluated these results in a randomized trial of 20 patients using the same radiotherapy and chemotherapy schedule, with and without amifostine (825 mg/m2 before each cisplatin infusion).[76] Although not statistically significant, decreases in grade 3 neutropenia and grade 2 neurotoxicity were seen. Of note, a majority of patients in the amifostine arm developed hypocalcemia during treatment.
Subcutaneous daily amifostine has been investigated as an alternative to IV amifostine. Koukourakis and colleagues reported on 140 patients with various malignancies, including 40 with pelvic malignancies, randomized to receive radiation with or without 500 mg subcutaneous amifostine given 20 minutes prior to radiation.[77] No chemotherapy was given, and patients with gynecologic malignancies received 50 Gy EBRT followed by brachytherapy (total dose: 70 Gy to point A). Among all patients with pelvic malignancies, grade 2 or higher mucosal toxicities (13% vs 50%) and treatment delays longer than 3 days were significantly decreased (P < .05).
Currently, the RTOG is investigating the effect of amifostine in patients with positive high common-iliac or para-aortic lymph nodes who require extended-field irradiation with concurrent cisplatin (RTOG 0116).[78] In the first arm of this two-part, phase I/II study, patients received external-beam radiation (45 Gy) and brachytherapy (point A total dose of 85 Gy LDR equivalent) with concurrent weekly cisplatin (no amifostine). Excluding grade 3 leukopenia, the acute grade 3/4 toxicity rate from the first arm was 81%.[79] Four of five patients with a grade 4 late toxicity had bowel complications necessitating surgical intervention. The second arm, in which patients receive the same radiation with cisplatin along with daily subcutaneous amifostine (500 mg) prior to each radiation fraction, is currently accruing. The trial is expected complete accrual sometime in 2006.
Conclusions
The hope of cure for patients diagnosed with cervical cancer was improved in 1999, with the advent of chemoradiation. Since that time, there continues to incremental, yet substantial, improvements in therapy. Persistent vigilance is required to minimize long-term complications. Continued research is needed to determine which of these approaches will become an integral part of establishing a new standard of care.
References:
1. American Cancer Society: Cancer Facts & Figures 2005 (www.cancer.org/downloads/STT/CAFF2005f4PWSecured.pdf).
2. NCCN Practice Guidelines in Oncology-Cervical Cancer, v.2.2006 (www.nccn.org/professionals/physician_gls/PDF/cervical.pdf).
3. Fagundes H, Perez CA, Grigsby PW, et al: Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 24:197-204, 1992.
4. Souhami L, Gil R, Allan S, et al: A randomized trial of chemotherapy followed by pelvic radiation therapy in stage IIIB carcinoma of the cervix. Int J Radiat Oncol Biol Phys 9:970-997, 1991.
5. Cardena J, Olguin A, Figuerosa F, et al: Neoadjuvant chemotherapy (CT) + radiotherapy (RT) vs RT in stage IIB, III carcinoma of the cervix: A cooperative study of the French oncology centers (abstract 743). Proc Am Soc Clin Oncol 11:1992.
6. Sardi J, Sananes C, Giaroli A, et al: Results of a prospective randomized trial with neoadjuvant chemotherapy in stage IB, bulky carcinoma of the cervix. Gynecol Oncol 49:153-155, 1993.
7. Tattersall MH, Ramirez C, Coppleson M: A randomized trial comparing platinum based chemotherapy followed by radiotherapy vs. radiotherapy alone in patients with locally advanced cervical cancer. Int J Gynecol Cancer 2:244-251, 1993.
8. Kumar L, Jaushal R, Nandy M, et al: Chemotherapy followed by radiotherapy vs. radiotherapy alone in locally advanced cervical cancer: A randomized study. Gynecol Oncol 54:307-315, 1994.
9. Leborgne F, Leborgne JH, Doldan R, et al: Induction chemotherapy and radiotherapy of advanced cancer of the cervix: A pilot study and phase III randomized trial. Int J Radiat Oncol Biol Phys 37:343-350, 1997.
10. Tattersall MH, Lorvidhaya V, Vootiprux V, et al: Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. J Clin Oncol 12:444-451, 1995.
11. Morris M, Eifel P, Jiandong L, et al: Pelvic radiation with concurrent chemotherapy compared with pelvic and paraaortic radiation for high risk cervical cancer. N Engl J Med 340:1137-1143, 1999.
12. Keys HM, Bundy B, Stehman FB, et al: Cisplatin, radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340:1154-1161, 1999.
13. Rose P, Bundy B, Watkins E, et al: Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340:1144-1153, 1999.
14. Peters WA III, Liu PY, Rolland JB, et al: Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606-1613, 2000.
15. Whitney CW, Sause W, Bundy B, et al: Randomized comparison of fluorouracil plus cisplatin vs hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 17:1339-1348, 1999.
16. Pearcey R, Brundage M, Drouin P, et al: Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell carcinoma of the cervix. J Clin Oncol 20:966-972, 2002.
17. National Cancer Institute Clinical Announcement (direct mailing): Concurrent Chemoirradiation for Cervical Cancer, pp 1-5, February 1999.
18. Landoni F, Maneo A, Colombo A, et al: Randomized study of radical surgery versus radiotherapy for stage IB-IIA cervical cancer. Lancet 350:535-540, 1997.
19. Nag S, Chao C, Erickson B, et al: The American Brachytherapy Society recommendations for low-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 52:33-48, 2002.
20. Grinsky T, Rey A, Roche B, et al: Overall treatment time in advanced cervical carcinoma: A critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys 27:1051-1056, 1993.
21. Perez CA, Grigsby PW, Castro-Vita H, et al: Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys 32:1275-1288, 1995.
22. Lanciano RM, Pajak TF, Martz K, et al: The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: A patterns-of-care study. Int J Radiat Oncol Biol Phys 25:391-397, 1993.
23. Montana GS, Fowler WC, Varra MA, et al: Carcinoma of the cervix, stage III: Results of radiation therapy. Cancer 57:148-154, 1986.
24. Perez CA, Breaux S, Madoc-Jones H, et al: Radiation therapy alone in the treatment of carcinoma of the uterine cervix: I. Analysis of tumor recurrence. Cancer 51:1393-1402, 1983.
25. Lanciano RM, Won M, Coia LR, et al: Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: A final report of the 1973 and 1978 Patterns of Care studies. Int J Radiat Oncol Biol Phys 20:667-676,
1991.
26. Hanks GE, Kerring DF, Kramer S: Patterns of Care outcome studies. Results of the national practice in cancer of the cervix. Cancer 51:959-967, 1983.
27. Coia L, Won M, Lanciano R, et al: The Patterns of Care outcome study for cancer of the uterine cervix. Results of the second national practice survey. Cancer 66:2451-2456, 1990.
28. Montana GS, Hanlon AL, Brickner TL, et al: Carcinoma of the cervix: Patterns of Care studies. Review of 1978, 1983, and 1988-1989 surveys. Int J Radiat Oncol Biol Phys 32:1481-1486, 1995.
29. Montana GS, Martz KL, Hanks GE: Patterns and sites of failure in cervix cancer treated in the USA in 1978. Int J Radiat Oncol Biol Phys 20:87-93, 1991.
30. Corn BW, Hanlon AL, Pajak TF, et al: Technically accurate intracavitary insertions improve pelvic control and survival among patients with locally advanced carcinoma of the uterine cervix. Gynecol Oncol 53:294-300, 1994.
31. Tod M, Meredith W: A dosage system for use in the treatment of cancer of the uterine cervix. Br J Radiol 11:809-824, 1938.
32. Tod M, Meredith W: Treatment of cancer of the cervix uteri-A revised Manchester method. Br J Radiol 26:252-257, 1953.
33. Nag S, Erickson B, Thomadsen B, et al: The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Biol Phys 48:201-211, 2000.
34. Akine Y, Tokita N, Ogino T, et al: Dose equivalence for high-dose rate to low-dose rate intracavitary irradiation in the treatment of cancer of the uterine cervix. Int J Radiat Oncol Biol Phys 19:1511-1514, 1990.
35. Arai T, Nakano T, Morita S, et al: High-dose-rate remote afterloading intracavitary radiation therapy for cancer of the cervix. Cancer 69:175-180, 1992.
36. Cikaric S: Radiation therapy of cervical carcinoma using either HDR or LDR afterloading: Comparison of 5-year results and complications. Strahlenther Onkol 82(suppl): 119-122, 1988.
37. Fu KK, Phillips TL: High-dose-rate vs. low-dose rate intracavitary brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 19:791-796, 1990.
38. Glaser FH: Comparsion of HDR afterloading with 192Ir versus conventional radium therapy in cervix cancer: 5-year results and complications. Strahlenther Onkol 82(suppl):106-113, 1988.
39. Kupiers T: High dose-rate intracavitary irradiation: Results of treatment, in Mould RF (ed): Brachytherapy, pp 169-175. Leersum,
The Netherlands, Nucleotron Trading BV,
1985.
40. Orton CG, Seyedsadr M, Somnay A: Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys 21:1425-1434, 1991.
41. Patel FD, Sharma SC, Negi PS, et al: Low dose rate vs HDR brachytherapy in the treatment of carcinoma of the uterine cervix: A clinical trial. Int J Radiat Oncol Biol Phys 28:335-341, 1994.
42. Sarkaria JN, Petereit DG, Stitt JA, et al: A comparison of the efficacy and complication rates of low-dose rate vs high-dose rate brachytherapy in the treatment of uterine cervical carcinoma. Int J Radiat Oncol Biol Phys 9:351-356, 1994.
43. Shigematsu Y, Nishiyama K, Masaki N, et al: Treatment of carcinoma of the uterine cervix by remotely controlled afterloading intracavitary radiotherapy with HDR: A comparative study with a low-dose rate system. Int J Radiat Oncol Biol Phys 9:351-356, 1983.
44. Teshima T, Inoue T, Ikeda H, et al: High-dose rate and low-dose rate intracavitary therapy for carcinoma of the uterine cervix. Cancer 72:2409-2414, 1993.
45. Orton CG: High and low dose brachytherapy for cervical cancer. Acta Oncol 37:117-127, 1998.
46. Petereit DG, Pearcey R: Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer: Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys 43:359-366, 1999.
47. Lurain JR: Newer diagnostic approaches to the evaluation of gynecologic malignancies. Obstet Gynecol Surv 37:437-448, 1982.
48. Scheidler J, Hricak H, Yu KK, et al: Radiological evaluation of lymph node metastases in patients with cervical cancer. A meta-analysis. JAMA 278:1096-1101, 1997.
49. Heller PB, Malfetano JH, Bundy BN, et al: Clinical-pathologic study of stage IIB, III, and IVA carcinoma of the cervix: Extended diagnostic evaluation for paraaortic node metastasis-A Gynecologic Oncology Group study. Gynecol Oncol 38:425-430, 1990.
50. Rose PG, Adler LP, Rodriguez M, et al: Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: A surgicopathologic study. J Clin Oncol 17:41-45, 1999.
51. Reinhardt MJ, Ehritt-Braun C, Vogelgesang D, et al: Metastatic lymph nodes in patients with cervical cancer: Detection with MR imaging and FDG PET. Radiology 218:776-782, 2001.
52. Grigsby PW, Siegel BA, Dehdashti F: Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol 19:3745-3749, 2001.
53. Lin LL, Mutic S, Malyapa RS, et al: Sequential FDG brachytherapy treatment planning in carcinoma of the cervix. Int J Radiat Biol Phys 63:1494-1501, 2005.
54. Umesaki N, Tanaka T, Miyama M, et al: Early diagnosis and evaluation of therapy in postoperative recurrent cervical cancer by positron emission tomography. Oncol Rep 7:53-56, 2000.
55. Stock R, Chan K, Terk M, et al: A new technique for performing Syed-Neblett template interstitial implants for gynecologic malignancies using transrectal-ultrasound guidance. Int J Radiat Oncol Biol Phys 37:819-825, 1997.
56. Erickson B, Albano K, Gillin M: CT-guided interstitial implantation of gynecologic malignancies. Int J Radiat Oncol Biol Phys 36:699-709, 1996.
57. Mutic S, Grigsby P, Low D, et al: PET-guided three-dimensional treatment planning of intracavitary gynecologic implants. Int J Radiat Oncol Biol Phys 52:1104-1110,
2002.
58. Nag S, Cardenes H, Chang S, et al: Proposed guidelines for image-based intracavitary brachytherapy for cervical carcinoma: Report from Image-Guided Brachytherapy Group. Int J Radiat Oncol Biol Phys 60:1160-1172, 2004.
59. Kirisits C, Potter R, Lang S, et al: Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys 62:901-911, 2005.
60. Haie-Meder C, Potter R, Van Limbergen E, et al: Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 74:235-245, 2005.
61. Potter R, Haie-Meder C, Van Limbergen E, et al: Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 78:67-77, 2006.
62. Hall EJ: Hyperthermia, in Hall EJ: Radiobiology for the Radiologist, 5th ed, pp 495-520. Philadelphia, Lippincott Williams & Wilkins, 2000.
63. van der Zee J, Gonzalez DG, van Rhoon GC, et al: Comparison of Radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: A prospective, randomised, multicentre trial. Lancet 355:1119-1125, 2000.
64. Vasanathan A, Mitsumori M, Park JH, et al: Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 61:145-153, 2005.
65. Westermann AM, Jones EL, Schem B, et al: First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer 104:763-770, 2005.
66. Portelance L, Chao KS, Grigsby PW, et al: Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys 51:261-266, 2001.
67. Mutic S, Malyapa RS, Grigsby PW, et al: PET-guided IMRT for cervical carcinoma with positive para-aortic lymph nodes-a dose-escalation treatment planning study. Int J Radiat Oncol Biol Phys 55:28-35, 2003.
68. Adli M, Mayr NA, Kaiser HS, et al: Does prone positioning reduce small bowel dose in pelvic radiation with intensity-modulated radiotherapy for gynecologic cancer? Int J Radiat Oncol Biol Phys 57:230-238, 2003.
69. Heron DE, Gerszten K, Selvaraj RN, et al: Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: a comparative dosimetric study of dose-volume histograms small star, filled. Gynecol Oncol 91:39-45, 2003.
70. Ahmed RS, Kim RY, Duan J, et al: IMRT dose escalation for positive para-aortic lymph nodes in patients with locally advanced cervical cancer while reducing dose to bone marrow and other organs at risk. Int J Radiat Oncol Biol Phys 60:505-512, 2004.
71. Mundt AJ, Lujan AE, Rotmensch J, et al: Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 52:1330-1337, 2002.
72. Grdina DJ, Kataoka Y, Murley JS: Amifostine: Mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact 16:237-279, 2000.
73. Capizzi RL, Oster W: Chemoprotective and radioprotective effects of amifostine: An update of clinical trials. Int J Hematol 72:425-535, 2000.
74. Dorr RT: Radioprotectants: Pharmacology and clinical applications of amifostine. Semin Radiat Oncol 8(4 suppl 1):10-13, 1998.
75. Wadler S, Beitler JJ, Rubin JS, et al: Pilot trial of cisplatin, radiation, and WR2721 in carcinoma of the uterine cervix: A New York Gynecologic Group Study. J Clin Oncol 11:1511-1516, 1993.
76. Gallardo D, Mohar A, Calderillo G, et al: Cisplatin, radiation, and amifostine in carcinoma of the uterine cervix. Int J Gynecol Cancer 9:225-230, 1999.
77. Koukourakis MI, Kyrias G, Kakolyris S, et al: Subcutaneous administration of amifostine during fractionated radiotherapy: A randomized phase II study. J Clin Oncol 18:2226-2233, 2000.
78. Small W Jr: The potential role of amifostine in the treatment of carcinoma of the uterine cervix: A review. Semin Radiat Oncol 12(1 suppl 1):68-74, 2002.
79. Small W Jr, Winter K, Levenback C, et al: Extended field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para-aortic or high common iliac lymph nodes: Results of arm 1 of RTOG 0116. Int J Radiat Oncol Biol Phys 63(suppl 2):S94, 2005.
80. Benedet JL, Odicino F, Maisonneuve P, et al: Carcinoma of the cervix uteri. J Epidemiol Biostat 6:7-43, 2001.