Cancer Management Chapter 2: Thyroid and parathyroid cancers
Endocrine malignancies, although relatively uncommon, are often difficult to diagnose and treat effectively. According to American Cancer Society (ACS) estimates, more than 39,000 new cases of endocrine neoplasms will be diagnosed in the United States in 2009, and approximately 2,470 deaths will result from these cancers. This chapter will focus on thyroid and parathyroid cancers. (A discussion of carcinoid tumors, insulinomas, gastrinomas, and other gastrointestinal neuroendocrine tumors, as well as adrenocortical cancer, can be found in chapter 11.)
Endocrine malignancies, although relatively uncommon, are often difficult to diagnose and treat effectively. According to American Cancer Society (ACS) estimates, more than 39,000 new cases of endocrine neoplasms will be diagnosed in the United States in 2009, and approximately 2,470 deaths will result from these cancers. This chapter will focus on thyroid and parathyroid cancers. (A discussion of carcinoid tumors, insulinomas, gastrinomas, and other gastrointestinal neuroendocrine tumors, as well as adrenocortical cancer, can be found in chapter 11.)
THYROID CANCER
Thyroid cancer is the most common endocrine cancer. The number of deaths from thyroid cancer estimated for 2009 is 1,630, or 4.3% of all new thyroid cancer cases.
The prevalence rate for occult thyroid cancers found at autopsy is 5% to 10%, except in Japan and Hawaii, where the rate can be as high as 28%. Autopsy rates do not correlate with clinical incidence.
The prevalence of thyroid nodules in the general population is 4% to 7%, with nodules being more common in females than males. The prevalence of thyroid cancer in a solitary nodule or in multinodular thyroid glands is 10% to 20%; this increases with irradiation of the neck in children and older men (see section on “Etiology and risk factors”).
Tumor types
Thyroid cancer is classified into four main types according to its morphology and biologic behavior: papillary, follicular, medullary, and anaplastic. Differentiated (papillary and follicular) thyroid cancers account for > 90% of thyroid malignancies and constitute approximately 0.8% of all human malignancies. Medullary thyroid cancers represent 3% to 5% of all thyroid neoplasms. About 75% of patients with medullary cancer have a sporadic form of the disease, whereas the remaining 25% have inherited disease. Anaplastic carcinoma represents < 3% of all thyroid carcinomas.
Papillary thyroid carcinoma is the most common subtype and has an excellent prognosis. Most papillary carcinomas contain varying amounts of follicular tissue. When the predominant histology is papillary, the tumor is considered to be a papillary carcinoma. Because the mixed papillary-follicular variant tends to behave like a pure papillary cancer, it is treated in the same manner and has a similar prognosis.
Papillary tumors arise from thyroid follicular cells, are unilateral in most cases, and are often multifocal within a single thyroid lobe. They vary in size from microscopic to large cancers that may invade the thyroid capsule and infiltrate into contiguous structures. Papillary tumors tend to invade the lymphatics, but vascular invasion (and hematogeneous spread) is uncommon.
Up to 40% of adults with papillary thyroid cancer may present with regional lymph node metastases, usually ipsilateral. Distant metastases occur, in decreasing order of frequency, in the lungs, bones, and other soft tissues. Older patients have a higher risk for locally invasive tumors and for distant metastases. Children may present with a solitary thyroid nodule, but cervical node involvement is more common in this age group; up to 10% of children and adolescents may have lung involvement at the time of diagnosis.
Follicular thyroid carcinoma is less common than papillary thyroid cancer, occurs in older age groups, and has a slightly worse prognosis. Follicular thyroid cancer can metastasize to the lungs and bones, often retaining the ability to accumulate radioactive iodine (which can be used for therapy). Metastases may be appreciated many years after the initial diagnosis.
Follicular tumors, although frequently encapsulated, commonly exhibit microscopic vascular and capsular invasion. Microscopically, the nuclei tend to be large and have atypical mitotic figures. There is usually no lymph node involvement.
Follicular carcinoma can be difficult to distinguish from its benign counterpart, follicular adenoma. This distinction is based on the presence or absence of capsular or vascular invasion, which can be evaluated after surgical excision but not by fine-needle aspiration (FNA).
Thyroglobulin, normally synthesized in the follicular epithelium of the thyroid, is present in well-differentiated papillary and follicular carcinomas and infrequently in anaplastic carcinomas but not in medullary carcinomas. Therefore, thyroglobulin immunoreactivity is considered to be indicative of a follicular epithelial origin.
Hrthle cell, or oxyphil cell, carcinoma is a variant of follicular carcinoma. Hrthle cell carcinoma is composed of sheets of Hrthle cells and has the same criteria for malignancy as does follicular carcinoma. Hrthle cell carcinoma is thought to have a worse outcome than follicular carcinoma and is less apt to concentrate radioactive iodine.
Medullary thyroid carcinoma originates from the C cells (parafollicular cells) of the thyroid and secretes calcitonin. Secretory diarrhea and flushing, related to calcitonin secretion, can be clinical features of advanced medullary thyroid carcinoma. On gross examination, most tumors are firm, grayish, and gritty.
Sporadic medullary thyroid carcinoma usually presents as a solitary thyroid mass; metastases to cervical and mediastinal lymph nodes are found in half of patients and may be present at the time of initial presentation. Distant metastases to the lungs, liver, bones, and adrenal glands most commonly occur late in the course of the disease.
Hereditary medullary thyroid carcinoma typically presents as a bilateral, multifocal process. Histologically, hereditary medullary carcinoma of the thyroid does not differ from the sporadic form. However, the hereditary form is frequently multifocal, and it is common to find areas of C-cell hyperplasia in areas distant from the primary carcinoma. Another characteristic feature of hereditary medullary carcinoma is the presence of amyloid deposits.
There are three hereditary forms: familial medullary thyroid carcinoma; multiple endocrine neoplasia type 2A (MEN-2A), characterized by medullary thyroid cancer, pheochromocytomas, and hyperparathyroidism; and multiple endocrine neoplasia type 2B (MEN-2B), characterized by medullary thyroid cancer, marfanoid habitus, pheochromocytomas, and neuromas. These syndromes are associated with germ-line mutations of the RET proto-oncogene, which codes for a receptor tyrosine kinase (RTK). Hereditary medullary thyroid carcinoma is inherited as an autosomal-dominant trait with high penetrance and variable expression. In addition, approximately 40% of sporadic medullary thyroid carcinomas contain somatic RET mutations, which may represent potential therapeutic targets. (For a discussion of genetic testing to screen for RET mutations in MEN kindreds, see section on “Diagnostic workup.”)
Anaplastic carcinoma Anaplastic tumors are high-grade neoplasms characterized histologically by a high mitotic rate and lymphovascular invasion. Aggressive invasion of local structures is common, as are lymph node metastases. Distant metastases tend to occur in patients who do not succumb early to regional disease. Occasional cases of anaplastic carcinoma have been shown to arise from preexisting differentiated thyroid carcinoma or in a preexisting goiter.
Other tumor types Lymphomas of the thyroid account for < 5% of primary thyroid carcinomas. Other tumor types, such as teratomas, squamous cell carcinomas, and sarcomas, may also rarely cause primary thyroid cancers.
Epidemiology
Age and gender Most patients are between the ages of 25 and 65 years at the time of diagnosis of thyroid carcinoma. Women are affected more often than men (2:1 ratio for the development of both naturally occurring and radiation-induced thyroid cancer).
Etiology and risk factors
Differentiated thyroid cancer
Therapeutic irradiation External low-dose radiation therapy to the head and neck during infancy and childhood, frequently used between the 1940s and 1960s for the treatment of a variety of benign diseases, has been shown to predispose an individual to thyroid cancer. The younger a patient is at the time of radiation exposure, the higher is the subsequent risk of developing thyroid carcinoma. Also, as mentioned previously, women are at increased risk of radiation-induced thyroid cancer. There is a latency period ranging from 10 to 30 years from the time of low-dose irradiation to the development of thyroid cancer.
As little as 11 cGy and as much as 2,000 cGy of external radiation to the head and neck have been associated with a number of benign and malignant diseases. It was once thought that high-dose irradiation (> 2,000 cGy) to the head and neck did not increase the risk of neoplasia. However, it has been shown that patients treated with mantle-field irradiation for Hodgkin lymphoma are at increased risk of developing thyroid carcinoma compared with the general population, although they are more likely to develop hypothyroidism than thyroid cancer.
Radiation-associated thyroid cancer has a natural history and prognosis identical to sporadic thyroid cancer.
The presence of a BRAF mutation in FNA biopsy specimens of papillary thyroid carcinoma is highly predictive of extrathyroidal extension, thyroid capsular invasion, and lymph node metastases and an increased risk for persistent or recurrent disease over 3 years of follow-up. This test may be useful in directing initial surgical management and adjuvant therapy
(Xing M et al: J Clin Oncol 27:2977–2982, 2009)
.
Other factors Besides radiation-induced thyroid cancer, there are only sparse data on the etiology of differentiated thyroid cancer. There has been intensive research on distinguishing molecular factors important for cell differentiation, growth, and motility. Considerable attention has focused on BRAF, a member of the RAF family of serine/threonine kinases that mediates cellular responses to growth-promoting signals via the RAS-RAF-MEK-MAPK signaling pathway. BRAF mutations so far have only been documented in papillary thyroid carcinoma (45%) and papillary thyroid carcinoma-derived anaplastic thyroid carcinoma (25%). Over the past few years, BRAF mutations have been implicated as potential prognostic factors and therapeutic targets.
Signs and symptoms
Most thyroid cancers present as asymptomatic thyroid nodules. Patients may feel pressure symptoms from nodules as they begin to increase in size. A change in the voice can be caused by a thyroid cancer or benign goiter. The voice change usually occurs when there is compression of the larynx or invasion of the recurrent laryngeal nerve.
On physical examination, a thyroid nodule that is hard or firm and fixed may represent a cancer. The presence of palpable enlarged nodes in the lateral neck, even in the absence of a palpable nodule in the thyroid gland, could represent metastases to the lymph nodes.
Diagnostic workup
As mentioned previously, thyroid nodules are present in 4% to 7% of the general population and in a higher percentage of individuals who have had irradiation to the head and neck region. Most thyroid nodules are benign (colloid nodules or adenomas); therefore, it is important for the workup to lead to surgical resection for malignant nodules and to avoid unnecessary surgery for benign lesions. Although most solid nodules are benign, thyroid carcinomas usually present as solid nodules. A cystic nodule or a “mixed” (cystic-solid) lesion is less likely to represent a carcinoma and more likely to be a degenerated colloid nodule.
A recent study demonstrated that 97% of indeterminate thyroid nodule biopsies with mutations of BRAF, RAS, RET/PTC, and PAC8/PPAR-gamma had a malignant diagnosis after surgical resection. Molecular testing of a nodule for specific mutations can be useful in the analysis of an indeterminate FNA cytology
(Nikiforov YE et al: J Clin Endocrinol Metab 94:2092–2098, 2009)
.
History The history is important in the evaluation of thyroid nodules. If there is a history of irradiation to the head and neck, the risk of there being cancer in the nodule is higher (as great as 50%) than in nonirradiated patients (10% to 20% risk).
Age also is important in the evaluation of thyroid nodules. Nodules that occur in either the very young or the very old are more likely to be cancerous, particularly in men.
A new nodule or a nodule that suddenly begins to grow is worrisome as well.
FNA has become the initial diagnostic test for the evaluation of thyroid nodules. FNA can determine whether the lesion is cystic or solid. For solid lesions, cytology can yield one of three results: benign, malignant, or indeterminate. The accuracy of cytologic diagnosis from FNA is 70% to 80%, depending on the experience of the person performing the aspiration and the pathologist interpreting the cytologic specimen.
In a series of 98 “suspicious” FNAs, findings of cellular atypia (pleomorphism, enlarged nuclei, nuclear grooves, coarse or irregular chromatin, prominent or multiple nucleoli, or atypical or numerous mitotic figures) or follicular lesions with atypia were associated with malignancy 20% and 44% of the time, respectively. Follicular lesions without atypia have a 6.7% risk of malignancy. Core needle biopsy has been used as an alternative method for diagnosis. Some studies have shown the adequacy of sample may be greater with core biopsy than FNA. However, there are conflicting reports as to whether a core biopsy offers greater accuracy in the diagnosis of a thyroid nodule. Thus, as outlined in the 2006 guidelines by the American Thyroid Association, FNA biopsy remains the recommended procedure for evaluating thyroid nodules.
Imaging modalities Ultrasonographic and radionuclide (radioiodine and technetium) scans are also used in the evaluation of thyroid nodules.
Ultrasonography is now widely considered an essential tool in the assessment of thyroid nodules. The presence of certain features is associated with malignancy and can guide physicians in deciding which nodules should be biopsied. Although there is a decrease in cancer rate per nodule in patients with multiple nodules, the overall rate of thyroid cancer per patient is similar to that seen in patients with a solitary nodule.
Thyroid cancer is most often found in the dominant, or largest, nodule in multinodular glands; however, approximately one-third of the cases of cancer are found in nondominant nodules. Nodule size is a poor predictor for malignancy, as the likelihood of cancer has been shown to be the same regardless of nodule size.
A consensus statement from the Society of Radiologists in Ultrasound outlined various features of solitary nodules associated with thyroid cancer: microcalcifications, hypoechogenicity, irregular margins or no halo, solid composition, intranodule vascularity, and more tall than wide dimensions. No single feature has both high sensitivity and specificity; however, the combination of more than one factor can increase the likelihood of cancer. Other than characterization of thyroid nodules, ultrasonography can guide the FNA biopsy, which increases the diagnostic efficacy of the procedure. Additionally, ultrasonography can identify abnormal cervical lymph nodes, which should prompt a biopsy of the lymph node and possibly an ipsilateral thyroid nodule.
Thyroid isotope scans cannot differentiate absolutely a benign from a malignant nodule but can, based on the functional status of the nodule, assign a probability of malignancy. “Hot” thyroid nodules (ie, those that concentrate radioiodine) represent functioning nodules, whereas “cold” nodules are nonfunctioning lesions that do not concentrate the isotope. Most thyroid carcinomas occur in cold nodules, but only 10% of cold nodules are carcinomas. It is not necessary to operate on all cold thyroid nodules. CT or MRI scan of the neck may be appropriate in some cases.
Calcitonin level Medullary thyroid carcinomas usually secrete calcitonin, which is a specific product of the thyroid C cells (parafollicular cells). In patients who have clinically palpable medullary carcinoma, the basal calcitonin level is almost always elevated. In patients with smaller tumors or C-cell hyperplasia, the basal calcitonin level may be normal, but administration of synthetic gastrin (pentagastrin) or calcium results in marked elevation of calcitonin levels. The use of calcitonin levels as a tumor marker and stimulation screening in hereditary forms of medullary cancers has been largely replaced by genetic testing (see below).
Carcinoembryonic antigen (CEA) Serum CEA levels are elevated in patients with medullary thyroid cancer.
Ruling out pheochromocytoma Medullary thyroid carcinoma can be associated with MEN-2A, MEN-2B, or familial non-MEN. Both the MEN-2A and MEN-2B syndromes are characterized by medullary thyroid cancer and pheochromocytoma. Thus, in any patient with hereditary medullary thyroid carcinoma, it is imperative that the preoperative workup include a determination of 24-hour urinary catecholamine and metanephrine levels to rule out the presence of a pheochromocytoma. Fractionated plasma metanephrine levels have been demonstrated to have a high sensitivity and may be included in the initial assessment.
Genetic testing Germ-line mutations in the RET proto-oncogene are responsible for familial non-MEN medullary thyroid carcinoma in addition to MEN-2A and MEN-2B. DNA analysis performed on a peripheral blood sample is a highly reliable method for identifying the presence of a RET mutation. Approximately 95% of patients with a RET mutation will eventually develop medullary carcinoma of the thyroid; thus, prophylactic surgical treatment is recommended. The specific mutated codon of RET may correlate with the aggressiveness of medullary carcinoma of the thyroid. This should be considered when counseling affected individuals and their families regarding prophylactic thyroidectomy and the age at which to perform such surgery. Long-term data regarding the effectiveness of prophylactic thyroidectomy based on RET testing are scarce at this time. In a recent report of 50 patients (ages 19 years and younger) treated surgically after positive RET mutational analysis, 33 patients had carcinoma identified in the surgical specimen. At the time of the publication, 44 patients were found to be free of disease more than 5 years after surgery.
Recommended ages for prophylactic surgery range from within the first 6 months of life to 10 years of age, depending on the mutation. The prophylactic surgical procedure of choice is total thyroidectomy with or without central lymph node dissection.
Periodic determinations of stimulated calcitonin levels may help make the early diagnosis of medullary thyroid carcinoma in those who do not undergo surgery but will not always prevent the development of metastatic medullary thyroid carcinoma.
Screening
At this time, no organization recommends periodic screening for thyroid cancer using neck palpation or ultrasonography in average-risk, asymptomatic adults. However, the ACS recommends examination of the thyroid during a routine checkup, since this surveillance can result in case findings.
Staging and prognosis
Unlike most other cancers, in which staging is based on the anatomic extent of disease, the American Joint Committee on Cancer (AJCC) and International Union Against Cancer (UICC) staging of thyroid cancer also takes into consideration patient age at the time of diagnosis and tumor histology (
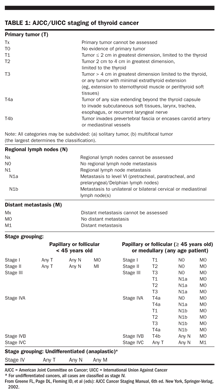
).
Differentiated thyroid cancers Recurrence and death following initial treatment of differentiated thyroid cancer can be predicted using a number of risk classification schemes. The most commonly used systems are the AMES (age, metastases, extent, and size) and AGES (age, grade, extent, and size) classifications.
Low-risk patients are generally those < 45 years of age with low-grade nonmetastatic tumors that are confined to the thyroid gland and are < 1 to 5 cm. Low-risk patients enjoy a 20-year survival rate of 97% to 100% after surgery alone.
High-risk patients are those ≥ 45 years old with a high-grade, metastatic, locally invasive tumor in the neck or with a large tumor. Large size is defined by some authors as > 1 cm and by other authors as > 2 or > 5 cm. The 20-year survival rate in the high-risk group drops to between 54% and 57%.
Intermediate-risk patients include young patients with a high-risk tumor (metastatic, large, locally invasive, or high grade) or older patients with a low-risk tumor. The 20-year survival rate in this group of patients is ~85%.
Medullary thyroid carcinoma is associated with an overall 10-year survival rate of 40% to 60%. When medullary carcinoma is discovered prior to becoming palpable, the prognosis is much better: patients with stage I medullary tumors (ie, tumors ≤ 2 cm or nonpalpable lesions detected by screening and provocative testing) have a 10-year survival rate of 95%.
Stage II medullary cancers (tumors > 2 but < 4 cm) are associated with a survival rate of 50% to 90% at 10 years. Patients who have lymph node involvement (stages III and IVA disease) have a 10-year survival rate of 15% to 50%. Unfortunately, approximately 50% of patients have lymph node involvement at the time of diagnosis.
When there are distant metastases (stages IVB and IVC), the long-term survival rate is compromised. In patients with metastatic medullary thyroid cancer, the disease often progresses at a very slow rate, and patients may remain alive with disease for many years.
Anaplastic thyroid cancer does not have a generally accepted staging system, and all patients are classified as having stage IV disease. Anaplastic carcinoma is highly malignant and has a poor 5-year survival rate (0% to 25%). Most patients die of uncontrolled local disease within several months of diagnosis.
Treatment
As most thyroid nodules are not malignant, it is important to differentiate malignant from benign lesions to determine which patients should undergo surgery. If the cytologic result from FNA indicates that the nodule is benign, which is the case most of the time, the nodule can be safely followed.
surgery
Malignant or indeterminate cytologic features are the main indications for surgery.
Malignant nodule
Differentiated thyroid cancer If the cytologic result shows a malignant lesion, thyroidectomy should be performed. There is significant debate in the literature regarding the extent of thyroid surgery for primary tumors confined to one lobe. The surgical options include total lobectomy, total lobectomy with contralateral subtotal lobectomy (subtotal thyroidectomy), or total thyroidectomy. The decision about which procedure to perform should be based on the risk of local recurrence and the anticipated use of radioactive iodine (see section on “Radioactive I-131”).
Most authorities agree that a good-risk patient (age < 45 years) with a 1-cm or smaller papillary thyroid cancer should undergo ipsilateral total lobectomy alone. Most experts also agree that total thyroidectomy (or at least subtotal thyroidectomy) is appropriate for high-risk patients with high-risk tumors. Intermediate-risk patients are treated with total lobectomy alone or total (or subtotal) thyroidectomy plus postoperative radioactive iodine. Preoperative neck imaging may help plan the surgery. Patients with radiation-induced thyroid malignancies can be treated similarly, as their cancers have a similar prognosis; however, a total thyroidectomy may be preferable in these patients because of the increased risk of multicentric tumors.
The neck should be palpated intraoperatively. If positive nodes are found, a regional lymph node dissection should be performed.
Medullary carcinoma Patients with medullary thyroid cancer should be treated with total thyroidectomy and a sampling of the regional nodes. If there is involvement of the nodes, a modified neck dissection should be performed (see section on “Lymph node dissection”). If the cancer is confined to the thyroid gland, the patient is usually cured. Postoperative adjuvant external irradiation may be used in certain circumstances (see section on “External radiation therapy”).
Anaplastic carcinoma A tracheostomy often is required in patients with anaplastic thyroid cancer because of compression of the trachea. If the tumor is confined to the local area, total thyroidectomy may be indicated to reduce local symptoms produced by the tumor mass. Radiation therapy is used to improve locoregional tumor control, often together with radiosensitizing chemotherapy.
Indeterminate or suspicious nodule
Indeterminate and suspicious FNA should be treated as possible cancers and removed for histologic evalution. The initial operation performed in most patients should be total lobectomy, which entails removal of the suspicious nodule, hemithyroid, and isthmus. There is no role for nodulectomy or enucleation of thyroid nodules. The specimen can be sent for frozen-section analysis during surgery. If frozen section is clearly benign no further resection is required.
Follicular lesion If frozen-section biopsy results indicate a follicular lesion in a patient who is a candidate for total thyroidectomy, and a decision cannot be made as to whether the lesion is benign or malignant, two options are available: (1) stop and wait for final confirmation of the diagnosis, which may require a future operation; or (2) proceed with subtotal or total thyroidectomy, which obviates the need for a later operation. The diagnosis of follicular carcinoma requires identification of vascular or capsular invasion, which may not be evident on frozen-section biopsy.
Hrthle cell carcinoma If the nodule is diagnosed as a Hrthle cell carcinoma, total thyroidectomy is generally recommended for all large (> 4 cm) invasive lesions. Small lesions can be managed with total lobectomy. However, controversy remains over the optimal treatment approach for this cancer.
Lymph node dissection
Therapeutic dissection Therapeutic central neck node dissection should be performed for medullary carcinomas and other thyroid neoplasms with nodal involvement. The dissection should include all the lymphatic tissue in the pretracheal area and along the recurrent laryngeal nerve and anterior mediastinum. If there are clinically palpable nodes in the lateral neck, a modified neck dissection is performed.
Prophylactic dissection There is no evidence that performing prophylactic neck dissection improves survival. Therefore, aside from patients with medullary thyroid cancer, who have a high incidence of involved nodes, only therapeutic neck dissection is indicated.
Removal of individual abnormal nodes (“berry picking”) is not advised when lateral neck nodes are palpable because of the likelihood of missing involved nodes and disrupting involved lymphatic channels.
Metastatic or recurrent disease
Survival rates from the time of the discovery of metastases (lung and bone) from differentiated thyroid cancer are less favorable than those associated with local recurrence (5-year survival rates of 38% and 50%, respectively). Survival also depends on whether the metastatic lesions take up I-131.
Surgery, with or without I-131 ablation (discussed below), can be useful for controlling localized sites of recurrence. Approximately half of patients who undergo surgery for recurrent disease can be rendered free of disease with a second operation.
Radioactive I-131
Uses in papillary or follicular thyroid carcinoma
There are two basic uses for I-131 in patients diagnosed with papillary or follicular thyroid carcinoma: ablation of normal residual thyroid tissue after thyroid surgery and treatment of thyroid cancer, either residual disease in the neck or metastasis to other sites in the body. It should be emphasized that patients with medullary, anaplastic, and most Hrthle cell cancers do not benefit from I-131 therapy.
Postoperative ablation of residual thyroid tissue should be considered in high-risk patients and patients with high-risk tumors. Ablation of residual normal thyroid tissue allows for the use of I-131 scans to monitor for future recurrence, possibly destroys microscopic foci of metastatic cancer within the remnant, and improves the accuracy of thyroglobulin monitoring.
Ablation must also be accomplished in patients with regional or metastatic disease prior to the use of I-131 for treatment, as the normal thyroid tissue will preferentially take up iodine compared with the cancer. Some states permit the use of I-131 for ablation and treatment on an outpatient basis, but administration is strictly governed by national guidelines, which minimize the risk of radiation exposure to the public.
Following surgery, the patient can be treated with liothyronine for a duration of 2 weeks. The TSH level should be determined approximately 4 to 6 weeks after surgery; in patients who undergo total or subtotal thyroidectomy, TSH levels will generally be > 50 μU/mL. A postoperative iodine scan can then be performed. If this scan documents residual thyroid tissue, an ablative dose of I-131 should be given. The patient should be advised not to undergo any radiographic studies with iodine during ablation therapy and to avoid seafood and vitamins or cough syrups containing iodine. Patients are prepared with a specific diet prior to the I-131 therapy. Iodine-123 may also be used in the postoperative setting. It may produce a better quality image than I-131 scans.
For patients who have contraindications for thyroid hormone withdrawal, administration of recombinant human thyroid-stimulating hormone (rTSH) is an alternative for preparation for radioiodine ablation of a post-surgical thyroid remnant. Currently, there are no long-term data ascertaining the maintenance of a low tumor recurrence rate using rTSH, which may have a quality-of-life advantage compared with thyroid hormone withdrawal and is the subject of a clinical trial.
In general, doses of I-131 up to 75 to 100 mCi will ablate residual thyroid tissue within 6 months following ingestion. In some patients, it may take up to 1 year for complete ablation to occur. Patients should be monitored following ablation, and when they become hypothyroid, hormone replacement therapy should be given until they are clinically euthyroid and TSH is suppressed. Recently, lower doses have been found to be effective, and some authors have recommended doses between 25 and 50 mCi, assuming they achieve euthyroid levels with TSH suppression to < 0.1 μU/mL.
Approximately 6 to 12 months after ablation of the thyroid remnant, a follow-up I-131 scan should be performed. Recombinant human thyrotropin alpha (Thyrogen) is now available. Patients may continue on thyroid replacement and receive two doses of thyrotropin prior to I-131 scanning; this approach can prevent the symptoms of hypothyroidism.
Treatment of residual cancer For disease in the tumor bed or lymph nodes that was not surgically resectable, an I-131 dose of 100 to 150 mCi is given. For disease in the lungs or bone, the I-131 dose is 200 to 250 mCi. Following this therapy, the patient is again put on thyroid hormone replacement, and adequate suppression is maintained by monitoring TSH levels.
Some clinicians advocate obtaining a repeat scan in 1 year, along with a chest x-ray, and repeating this procedure yearly until a normal scan is obtained. However, the frequency of repeat scans and the dose of I-131 are rather controversial and should be guided by the individual's risk profile.
Following thyroid remnant ablation, serum thyroglobulin measurements are useful in monitoring for recurrence. Since thyroglobulin in a patient receiving thyroid hormone replacement may be suppressed, a normal test may be incorrect ~10% of the time. In general, the presence of disease is accurately predicted by a thyroglobulin value > 5 ng/mL while the patient is in the suppressed state and by a value > 10 ng/mL in the hypothyroid state. However, measurable disease may not be identified in many patients. Whether or not they should be treated on the basis of the thyroglobulin value if the I-131 scan is normal is a subject of current debate. Any rise in the thyroglobulin level from the previous value should increase the suspicion of recurrent disease.
Various preclinical studies evaluating the effects of other novel therapies have shown promising results. PTC cell lines carrying a RET/PTC rearrangement or a BRAF mutation treated with CI-1040 (a MEK1/2 inhibitor) in vitro and in vivo demonstrated inhibition of PTC cell growth
(Henderson YC et al: Arch Otolaryngol Head Neck Surg 135:347–354, 2009)
. Treatment with AZD0530 (a Src inhibitor) inhibited the growth and invasion of human PTC and ATC cell lines
(Schweppe RE et al: J Clin Endocrinol Metab 94:2199–2203, 2009)
. Anaplastic and follicular thyroid cancer cell lines incubated with gefitinib (Iressa, an EGFR inhibitor) resulted in a dose-dependent decrease in colony formation. Additionally, ionization radiation combined with gefitinib reduced cell proliferation; thus, this combination may be promising for anaplastic and follicular thyroid carcinomas
(Lopez JP et al: Laryngoscope 118:1372–1376, 2008)
.
Neck ultrasonography is useful to evaluate locoregional tumor recurrence and should be performed at yearly intervals for 5 to 10 years after initial therapy depending upon the stage of disease. Continued monitoring is necessary, as late recurrence can occur. It should be pointed out that certain aggressive tumors may neither be radioactive iodine–avid nor synthesize thyroglobulin. PET scanning may contribute to localization of disease in some cases and may even carry prognostic value. PET/CT may be more useful than other imaging techniques; in a recent study, additional information was obtained with PET/CT in up to 67% of cases.
Side effects and complications
Acute effects The acute side effects of I-131 therapy include painful swelling of the salivary glands and nausea. Ibuprofen or other pain relievers are usually used to decrease salivary gland discomfort. Nausea may be treated with standard antiemetics.
Rarely, in patients with significant residual thyroid tissue, radioactive iodine may cause acute thyroiditis, with a rapid release of thyroid hormone. This problem can be treated with steroids and β-blockers.
Patients must also be cautioned not to wear contact lenses for at least 3 weeks following ingestion of I-131, as the tears are radioactive and will contaminate the lenses and possibly lead to corneal ulceration.
Bone marrow suppression and leukemia are potential long-term complications of I-131 therapy but are poorly documented and appear to be extremely rare. Patients should have a CBC count performed prior to ingestion of an I-131 dose to ensure adequate bone marrow reserve. They should also have yearly blood counts. Leukemia occurs rarely with doses of I-131 < 1,000 mCi.
Pulmonary fibrosis may be seen in patients with pulmonary metastases from papillary or follicular thyroid cancer who are treated with I-131. Those with a miliary or micronodular pattern are at greater risk, as a portion of normal lung around each lesion may receive radiation, leading to diffuse fibrosis.
Effects on fertility Data have documented an increase in follicle-stimulating hormone (FSH) levels in one-third of male patients treated with I-131. Changes in FSH after one or two doses of I-131 are generally transitory, but repeated doses may lead to lasting damage to the germinal epithelium. Sperm banking should be considered in male patients likely to receive cumulative doses of I-131 higher than 500 mCi.
The effects of I-131 on female fertility have been investigated. A published article showed no significant difference in the fertility rate in women receiving radioactive iodine. However, it is generally recommended to avoid pregnancy for 1 year after therapeutic I-131 administration.
No ill effects have been noted in the offspring of treated patients.
A recent update of a large study of women exposed to radioiodine for thyroid cancer treatment evaluated for post-treatment pregnancy history and offspring health found that exposure to I-131 of > 100 mCi was not associated with increased miscarriages, congenital malformations, or thyroid diseases or cancer in offspring
(Garsi JP et al: J Nucl Med 49:845–852, 2008)
.
external radiation therapy
Papillary or follicular thyroid cancer
There are a number of indications for external irradiation of papillary or follicular thyroid carcinoma. Surgery followed by radioactive iodine may be used for disease that extends beyond the capsule. However, if all gross disease cannot be resected, or if residual disease is not radioactive iodine–avid, external irradiation is used as part of the initial approach for locally advanced disease in older patients. The benefit of adjuvant external irradiation for cause-specific survival is inferred from institutional series. Intensity-modulated radiation therapy is associated with decreased severe late toxicities in an institutional series and provides the best target coverage in dosimetric studies.
Twice-daily radiation therapy may be of benefit over once-a-day treatment for local tumor control and possibly survival. Multimodality therapy with R0/R1 resection followed by doxorubicin with twice-daily radiation therapy had the highest rates of local tumor control and median survival
(Swaak-Kragten AT et al: Radiother Oncol 92:100–104, 2009)
.
Unresectable disease External irradiation is useful for unresectable disease extending into the connective tissue, trachea, esophagus, great vessels, and anterior mediastinum. For unresected disease, doses of 6,000 to 6,500 cGy are recommended. The patient should then undergo I-131 scanning, and, if uptake is detected, a dose of I-131 should be administered.
Recurrence after resection External irradiation may also be used after resection of recurrent papillary or follicular thyroid carcinoma that no longer shows uptake of I-131. In this situation, doses of 5,000 to 6,600 cGy are delivered to the tumor bed to prevent local recurrence. Multiple-field techniques and extensive treatment planning are necessary to deliver high doses to the target volume without the risk of significant complications.
Palliation of bone metastases External radiation therapy is useful in relieving pain from bone metastasis. If the metastasis shows evidence of I-131 uptake, the patient should be given a therapeutic dose of I-131 followed by local external radiation therapy to the lesion of up to 4,000 to 5,000 cGy. The use of intravenous bisphosphonate therapy has been shown to decrease the pain of bone metastasis and improve reported quality of life.
Anaplastic thyroid carcinoma
Anaplastic carcinoma of the thyroid is an exceptionally aggressive disease. It often presents as a rapidly expanding mass in the neck and may not be completely resected. External irradiation to full dose (6,000 to 6,500 cGy) may slow the progress of this disease but rarely controls it.
Chemoradiation therapy There are reports of the use of accelerated fractionation regimens of external irradiation (160 cGy twice daily to 5,700 cGy) with weekly doxorubicin in patients with anaplastic thyroid cancer, as well as reports of the combination of doxorubicin and cisplatin with external irradiation. These regimens have improved local tumor control but at the expense of increased toxicity. Unfortunately, the majority of patients die of local and/or distant recurrence.
Medullary thyroid carcinoma
External irradiation has been used for medullary thyroid cancer in the postoperative setting. Indications include positive surgical margins, gross residual disease, or extensive lymph node metastasis. The recommended dose is 5,000 to 7,000 cGy in 5 to 7 weeks.
Several molecular targeted tyrosine kinase inhibitors have shown promising results for thyroid cancer. Motesanib (inhibitor of VEGFR, PDGFR, and KIT) given in an open-label, single-arm, phase II study of 93 patients with progressive, differentiated thyroid cancer led to an objective response of 14% and stable disease in 67%. Median progression-free survival was 40 weeks. Treatment-related adverse events were diarrhea, hypertension, fatigue, and weight loss
(Sherman SI et al: N Engl J Med 359:331–342, 2008)
. Axitinib (inhibitor of VEGFR-1/2/3) given in an open-label, single-arm, phase II study of 60 patients with radioiodine-resistant, thyroid carcinoma of any histology demonstrated an objective response rate of 30% and stable disease in 38%
(Cohen EE et al: J Clin Oncol 26:4708–4713, 2008)
. The ongoing trial of sorafenib (Nexavar) in patients with thyroid carcinoma recently described that patients with a BRAF mutation had significantly longer progression-free survival times than those without a mutation (> 84 weeks vs 54 weeks;
(Brose MS et al: J Clin Oncol 27: abstract 6002, 2009)
.
Role of Medical therapy
Differentiated thyroid cancer
As mentioned previously, thyroid hormone is used to suppress TSH in most patients with differentiated thyroid cancer after surgery and I-131 (as appropriate) treatment. Greater TSH suppression has been associated with improved progression-free survival in patients with high-risk papillary thyroid carcinoma. Modest TSH suppression in patients with stage II disease yields similar results. Patients with stage I disease do not appear to have any change in outcomes based on the degree of TSH suppression.
Systemic chemotherapy is used for widespread disease, although reproducibly effective regimens have not been identified to date.
Over the past few years, molecularly targeted treatments have been studied in patients with advanced thyroid carcinoma no longer responsive to radioactive iodine. They include multitargeted tyrosine kinase inhibitors, a DNA methylation inhibitor, histone deacetylase inhibitors, a proteasome inhibitor, and a heat-shock protein-90 inhibitor. These agents are still being investigated in clinical trials.
According to recent 2008 National Comprehensive Cancer Network Guidelines, patients with metastatic differentiated or medullary thyroid carcinoma not amenable to surgery or radioiodine should be referred to a clinical trial investigating targeted therapies or recommended to off-label use of sorafenib, if a trial is not available to the patient, or maintained with best supportive care (www.nccn.org, Thyroid Carcinoma, v.1.2008).
Medullary thyroid carcinoma
In patients with medullary thyroid carcinoma, the usual treatment is surgery. Various oral, small molecule tyrosine kinase inhibitors have been investigated in patients with locally advanced, metastatic, or progressive hereditary and sporadic medullary thyroid carcinomas. The responses have been variable among agents and have consisted of partial response as the best outcome. However, the development of these novel agents and others offers much promise in the targeted treatment of metastatic medullary thyroid carcinoma, which currently has no effective cure. In patients with hereditary medullary carcinoma who have a coexisting pheochromocytoma, appropriate control of catecholamine hypersecretion should precede thyroid surgery.
An ongoing, multicenter, phase II trial of sunitinib (Sutent) in patients with progressive, differentiated, medullary and anaplastic thyroid cancer has demonstrated preliminary results of 80% with stable disease
(Ravaud A et al: J Clin Oncol 26: abstract 6058, 2008)
. Vandetanib (Zactima), a RET, VEGFR, and EGFR inhibitor, has been studied in hereditary and sporadic medullary thyroid carcinoma at 100 mg and 300 mg daily doses
(Haddad RI et al: J Clin Oncol 26: abstract 6018, 2008; Wells SA et al: J Clin Oncol 25: abstract 6018, 2007)
. A randomized, placebo-controlled phase III study of the 300-mg dose in hereditary/sporadic medullary thyroid cancer is ongoing. XL184, an inhibitor of RET, MET, VEGFR2, KIT, and FLT3, is currently in a phase I trial in which more than 22 patients have medullary thyroid carcinoma. Of the 16 evaluable patients on study for > 3 months, 50% achieved partial response, and the others have had stable disease; all had reductions in calcitonin and CEA
(Kurzrock R et al: Thyroid 18: abstract 77, 2008)
. Based on these encouraging results, an international phase III trial is under way.
Anaplastic thyroid carcinoma
As mentioned previously, the usual treatment for patients with resectable or localized anaplastic thyroid cancer is surgery. Like radiotherapy, chemotherapy is an important alternative approach, but further evaluation is needed to optimize its effectiveness. Patients with unresectable local tumors should be referred to clinical trials, treated with radiotherapy and chemotherapy, or maintained with best supportive care.
PARATHYROID CARCINOMA
Parathyroid carcinoma is a rare cause of hypercalcemia, accounting for < 2% of cases with primary hyperparathyroidism.
Epidemiology and etiology
The disease presents in midlife and occurs with similar frequency in both genders. The etiology of parathyroid carcinoma is obscure; an association with prior neck irradiation is not apparent. Parathyroid carcinoma can be associated with the hereditary hyperparathyroidism-jaw tumor syndrome, which is due to an inactivating mutation of the HRPT2 gene that encodes the parafibromin protein. In addition, somatic mutations of the HRPT2 gene have been demonstrated in sporadic parathyroid carcinomas (66% to 100%) but have not been seen with sporadic adenomas.
Signs and symptoms
Most patients with parathyroid cancer have symptomatic moderate to severe hypercalcemia (mean serum calcium level, 15 mg/dL) and high parathyroid hormone levels. They often present with a palpable neck mass. Unlike benign hyperparathyroidism, renal and bone abnormalities are more common in patients with parathyroid cancer.
Rarely, nonfunctioning tumors may present as neck masses; their clinical course is similar to that of functioning tumors. Clinical concern about parathyroid cancer should be raised in the presence of a palpable neck mass and severe hypercalcemia, recurrent hyperparathyroidism, or associated vocal cord paralysis.
Pathology
The principal features of parathyroid cancer include a trabecular pattern, mitotic figures, thick fibrous bands, and capsular or vascular invasion of disease. Other important features include lymphatic or hematogenous metastases and histologic evidence of tumor infiltration into the surrounding tissues (including macroscopic adherence or vocal cord paralysis).
Although cytologic evidence of mitoses is necessary to establish the diagnosis of carcinoma, mitotic activity alone is an unreliable indicator of malignancy. The only reliable microscopic finding of malignancy is invasion of surrounding structures or metastasis to lymph nodes or other organs.
Treatment
Surgical treatment of primary hyperparathyroidism
The diagnosis of parathyroid carcinoma is sometimes made during surgical exploration for primary hyperparathyroidism. Most surgeons advocate identification of all four parathyroid glands. In most cases, the upper glands can be found on the posterior aspect of the upper third of the thyroid lobe, just cephalad to the inferior thyroid artery and adjacent to the recurrent laryngeal nerve as it enters the larynx.
The inferior parathyroid glands are more variable in location. Most are found on the posterior or lateral aspect of the lower pole of the thyroid gland, but the inferior parathyroid glands may be ectopically placed in the superior or true mediastinum, often within the thymus.
The inferior and, less commonly, superior glands can be found in an ectopic location in the upper or lateral neck, adjacent to the esophagus, or within the carotid sheath.
Surgical exploration for primary hyperparathyroidism Most cases of primary hyperparathyroidism are caused by a single hyperfunctioning parathyroid adenoma. If the surgeon finds one (or occasionally two) enlarged abnormal gland(s) and the remaining glands are normal, the enlarged gland should be removed.
If four enlarged glands are found, indicating the rare case of primary parathyroid hyperplasia, subtotal parathyroidectomy including 3.5 glands should be performed. Consideration should be given to transplanting the remaining gland remnant to an ectopic location that would be easily accessible to the surgeon if hyperparathyroidism recurs.
If only normal glands are found at exploration, a missed adenoma in an ectopic location should be suspected. Thorough intraoperative neck and superior mediastinal exploration should be performed, and if the missing gland cannot be found, thymectomy and hemithyroidectomy should be performed to exclude an intrathymic or intrathyroidal adenoma. Localization studies, including CT/MRI or radionuclide imaging, should precede reexploration for a missed adenoma.
Intraoperative parathyroid hormone (ioPTH) levels are increasingly used to guide surgery for primary hyperparathyroidism. A 50% or greater decrease in the ioPTH level from the preexcision value to the 10-minute postexcision value is used as a predictor of successful surgery. The advent of ioPTH monitoring, coupled with preoperative localization studies (sestamibi scanning), has facilitated less invasive surgical techniques, such as minimally invasive parathyroidectomy. This has resulted in shorter average hospitalization stays and reduced postoperative recovery times.
The use of ioPTH with parathyroid hyperplasia requires more strict evaluation of ioPTH levels. Siperstein et al performed a prospective evaluation of ioPTH and bilateral neck exploration and found that up to 15% of cases will have additional “abnormal” glands that were not predicted by ioPTH or preoperative imaging. This study demonstrates the need for long-term follow-up of patients undergoing focused parathyroid surgery.
If parathyroid carcinoma is suspected, based on the severity of hyperparathyroidism or invasion of surrounding tissues by a firm parathyroid tumor, aggressive wide excision is indicated. This procedure should include ipsilateral thyroidectomy and en bloc excision of surrounding tissues as necessary.
Loss of parafibromin and Rb expression and overexpression of galectin-3 can be distinguishing features of parathyroid carcinoma vs other parathyroid tumors
(Fernandez-Ranvier GG et al: Cancer 115:334–344, 2009)
.
Patterns of recurrence of cancer The average time from initial surgery to the first recurrence of cancer is approximately 3 years but may be as long as 10 years. The thyroid gland is the usual site of involvement, with disease “seeding” in the neck a common pattern. Other sites of involvement include the recurrent nerve, strap muscles, esophagus, and trachea.
Distant metastases can be present at the time of initial surgery, or local spread to contiguous structures in the neck may be followed subsequently by distant metastases to the lungs, bone, and liver.
In a recent analysis, 85% of patients with parathyroid carcinoma were alive 5 years after diagnosis; death usually results from complications of the hypercalcemia rather than from the tumor burden.
Treatment of isolated metastases Isolated metastases should be aggressively resected to enhance survival and control hypercalcemia.
Medical therapy
Morbidity and mortality are generally caused by the effects of unremitting hypercalcemia rather than tumor growth. Medical treatment provides temporary palliation of hypercalcemia. Drugs used include bisphosphonates, such as pamidronate (60 to 80 mg every 4 to 6 days) or zoledronic acid (Zometa); calcitonin, 4 to 8 IU/kg every 6 to 12 hours; mithramycin (plicamycin [Mithracin]), 25 μg/kg every 4 to 6 days; and gallium nitrate (Ganite), 100 to 200 mg/m2/day IV for 5 days. Cinacalcet (Sensipar), a calcimimetic that targets the calcium-sensing receptor on parathyroid cells and reduces parathyroid hormone secretion, is an FDA-approved oral treatment of hypercalcemia associated with parathyroid carcinoma (up to 90 mg bid) in patients who do not respond to surgery or other medical treatments.
References:
SUGGESTED READING
on thyroid carcinoma
Bal CS, Kumar A, Pant GS: Radioiodine dose for remnant ablation in differentiated thyroid carcinoma: A randomized clinical trial in 509 patients. J Clin Endocrinol Metab 89:1666-1673, 2004.
David A, Blotta A, Rossi R, et al: Clinical value of different responses of serum thyroglobulin to recombinant human thyrotropin in the follow-up of patients with differentiated thyroid carcinoma. Thyroid 15:267â273, 2005.
Frates MC, Benson CB, Charboneau JW, et al: Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 237:794â800, 2005.
Garsi JP, Schlumberger M, Rubino C, et al: Therapeutic administration of 131-I for differentiated thyroid cancer: Radiation dose to ovaries and outcome of pregnancies. J Nucl Med 49:845â852, 2008.
Hall NC, Kloos RT: PET imaging in differentiated thyroid cancer: Where does it fit and how do we use it? Arq Bras Endocrinol Metab 51:793â805, 2007.
Jimenez C, Hu MI, Gagel RF: Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am 37:481â496, 2008.
Kloos RT, Eng C, Evans DB, et al: Medullary thyroid cancer: Management guidelines of the American Thyroid Association. Thyroid 19:565â612, 2009.
Meadows KM, Amdur RJ, Morris CG, et al: External beam radiotherapy for differentiated thyroid cancer. Am J Otolaryngol 27:24â28, 2006.
Sampson E, Brierley JD, Le LW, et al: Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 110:1451â1456, 2007.
Sawka AM, Lea J, Alshehri B, et al: A systematic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol (Oxf) 68:610â617, 2008.
Sherman SI: Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab 94:1493â1499, 2009.
Sherman SI, Angelos P, Ball DW, et al: Thyroid carcinoma. J Natl Compr Canc Netw 5:568â621, 2007.
Silverberg SJ, Rubin MR, Faiman C, et al: Cincalet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 92:3803â3808, 2007.
Torlontano M, Attard M, Crocetti U, et al: Follow-up of low risk patients with papillary thyroid cancer: Role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab 89:3402â3407, 2004.
United States Nuclear Regulatory Commission: Medical, industrial, and academic uses of nuclear materials: Regulations, guidance, and communications. Available at: www.nrc.gov.
Urhan M, Dadparvar S, Mavi A, et al: Iodine-123 as a diagnostic imaging agent in differentiated thyroid carcinoma: A comparison with iodine-131 post-treatment scanning and serum thyroglobulin measurement. Eur J Nucl Med Mol Imaging 34:1012â1017, 2007.
Verburg FA, de Keizer B, Lips CJ, et al: Prognostic significance of successful ablation with radioiodine of differentiated thyroid cancer patients. Eur J Endocrinol 152:33â37, 2005.
Ying AK, Huh W, Bottomley S, et al: Thyroid cancer in young adults. Semin Oncol 36:258â274, 2009.
On parathyroid carcinoma
Marcocci C, Cetani F, Rubin MR, et al: Parathyroid carcinoma. J Bone Miner Res 23:1869â1880, 2008.
Silverberg SJ, Rubin MR, Faiman C, et al: Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 92:3803â3808, 2007.
Siperstein A, Berber E, Mackey R, et al:Prospective evaluation of sestamibi scan, ultrasonography, and rapid PTH to predict the success of limited exploration of sporadic primary hyperparathyroidism. Surgery 136:872â880, 2004.