CancerNetwork® Recaps Gong’s Twitter Takeover at ASCO GI 2022
Jun Gong, MD, hosted a Twitter takeover during the 2022 Gastrointestinal Cancers Symposium where he discussed breaking presentations in a #CNRealtimeReport.
Jun Gong, MD, a medical oncologist of the Gastrointestinal Disease Research Group, Pancreatic Research Group, and Urologic Oncology Program in the Samuel Oschin Comprehensive Cancer Institute at Cedar Sinai

The 2022 Gastrointestinal Cancers Symposium hosted by the American Society of Clinical Oncology (ASCO) produced thought-provoking and breaking research across settings in pancreas, gastrointestinal tract, and hepatocellular cancers.
During all 3 days of the conference, CancerNetwork® held a Twitter takeover hosted by Jun Gong, MD, a medical oncologist of the Gastrointestinal Disease Research Group, Pancreatic Research Group, and Urologic Oncology Program in the Samuel Oschin Comprehensive Cancer Institute at Cedar Sinai. Gong spotlighted breaking abstracts during a #CNRealtimeReport, speaking to major takeaways and benefits of treatment.
First-Line Lenvatinib Plus Transarterial Chemoembolization
An important study Gong discussed was the phase 3 LAUNCH trial (NCT03905967) examining lenvatinib (Lenvima) plus transarterial chemoembolization (TACE) or lenvatinib monotherapy. “1L #lenvatinib + #TACE (w/repeat TACE allowed) vs lenvatinib in adv #HCC showing #OS benefit of combo vs len alone and improved ORR,” he wrote.
Tweet of the phase 3 LAUNCH trial
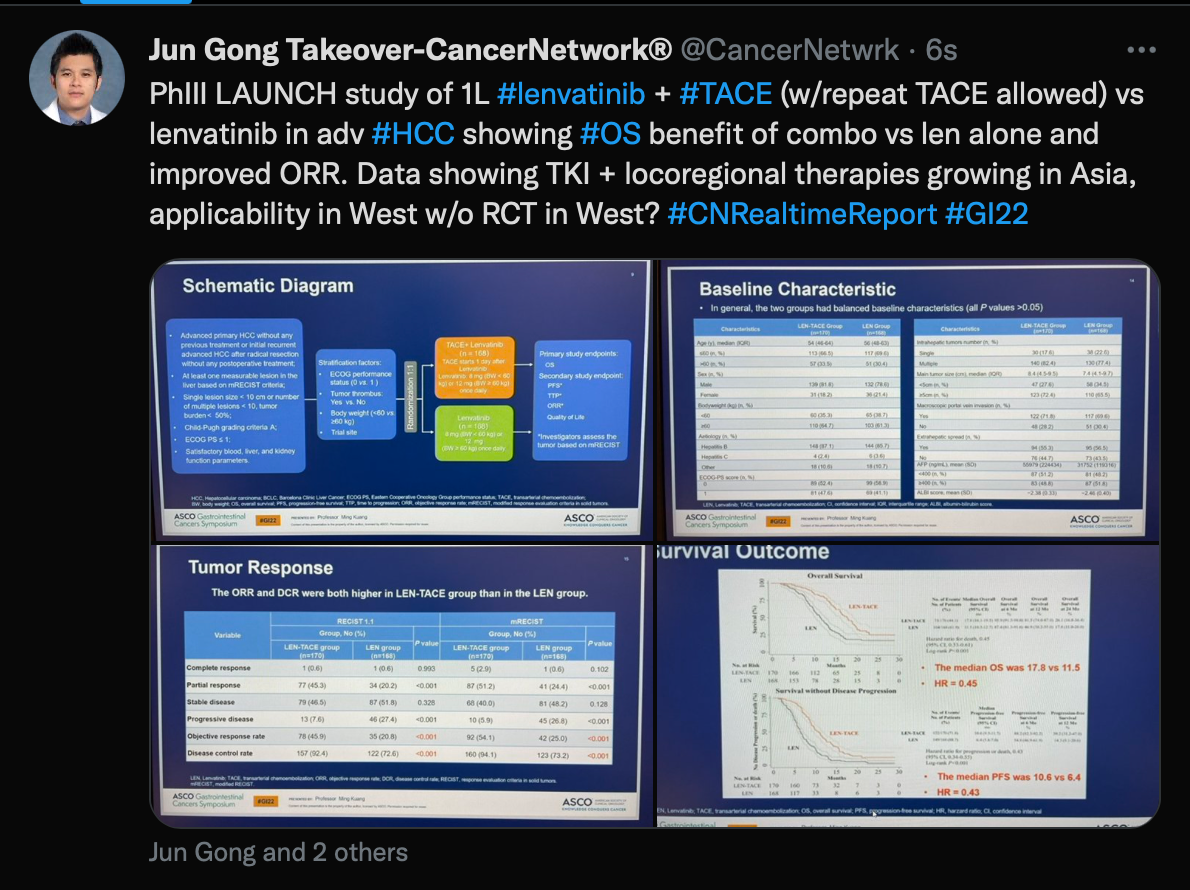
Patients had an objective response rate (ORR) of 45.9% with the TACE regimen vs 20.8% for lenvatinib alone (P <.001) via RECIST 1.1 criteria. The median overall survival (OS) in the TACE arm was 17.8 months vs 11.5 months with lenvatinib alone (HR, 0.45; 95% CI, 0.33-0.61; P <.001). Median progression-free survival (PFS) in each arm was 10.6 months and 6.4 months (HR, 0.43; 95% CI, 0.34-0.55; P <.001), respectively.
Additional findings showed a partial response (PR) rate of 45.3%, a stable disease rate of 46.5%, a progressive disease rate of 7.6%, and a disease control rate (DCR) of 92.4% in the TACE arm. In the monotherapy group, corresponding rates were 20.2%, 51.8%, 27.4%, and 72.6%.
CheckMate 649
Tweet of CheckMate 649
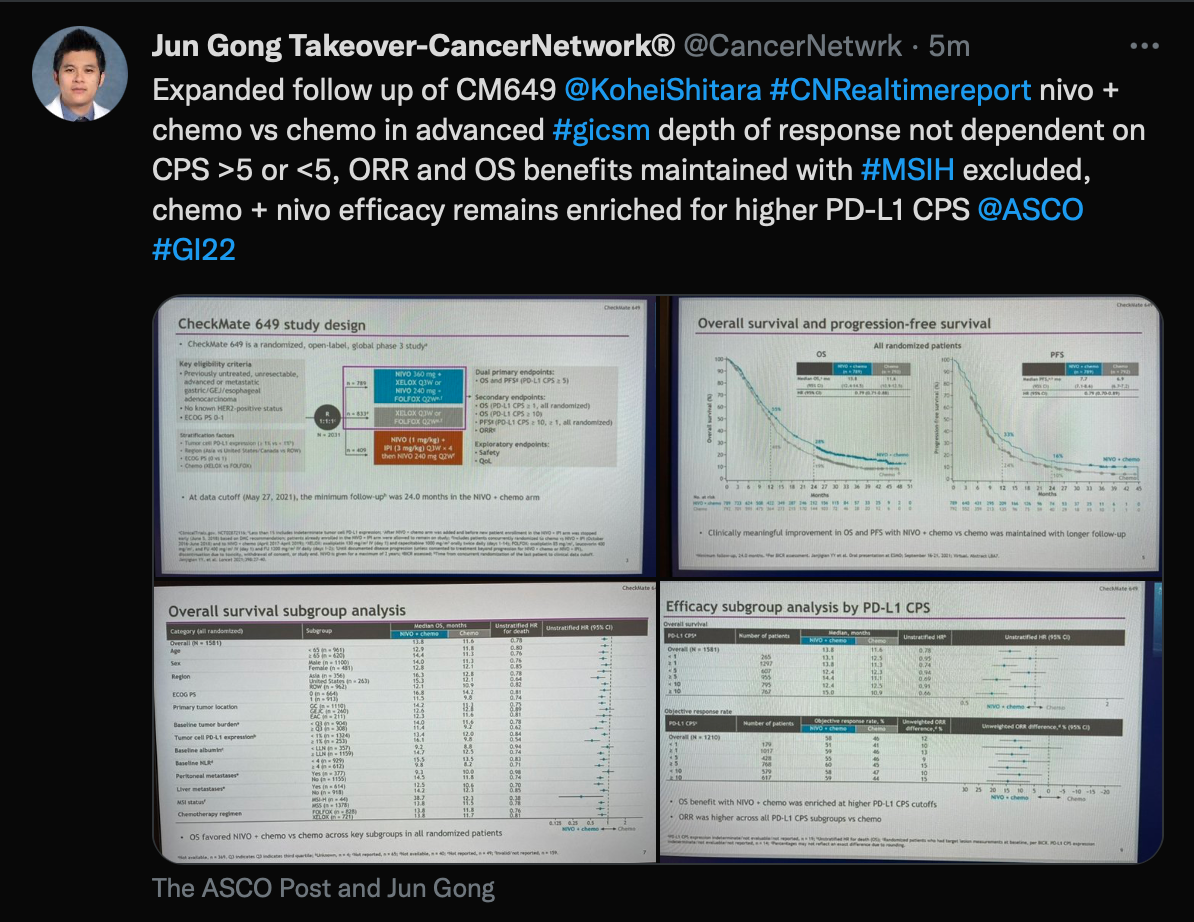
Next, Gong spoke about the CheckMate 649 trial (NCT02872116) regarding use of nivolumab (Opdivo) plus chemotherapy vs chemotherapy alone for patients with gastric or gastroesophageal junction cancer.
The median was 13.8 months (95% CI, 12.4-14.5) in the nivolumab group and 11.6 months (95% CI, 10.9-12.5) in the chemotherapy-alone group (HR, 0.79; 95% CI, 0.71-0.88). The median PFS in the nivolumab group was 7.7 months (95% CI, 7.1-8.6) compared with 6.9 months (95% CI, 6.7-7.2) with chemotherapy alone (HR, 0.79; 95% CI, 0.70-0.89).
Those in the nivolumab group saw a 25% reduction in the risk of death or disease progression on subsequent therapy (HR, 0.75; 95% CI, 0.67-0.84). The ORR for patients with a combined positive score less than 5 was 55% (95% CI, 48%-62%), with 7 patients having complete responses and 48 with PRs in the nivolumab group. The median duration of response was 7.7 months (95% CI, 6.2-9.9) in the nivolumab group vs 6.9 months (95% CI, 5.7-8.2) in the chemotherapy group.
No new safety signals were identified, and adverse effects remained consistent with the individual drug components.
TOPAZ-1
Tweet of the TOPAZ-1 trial
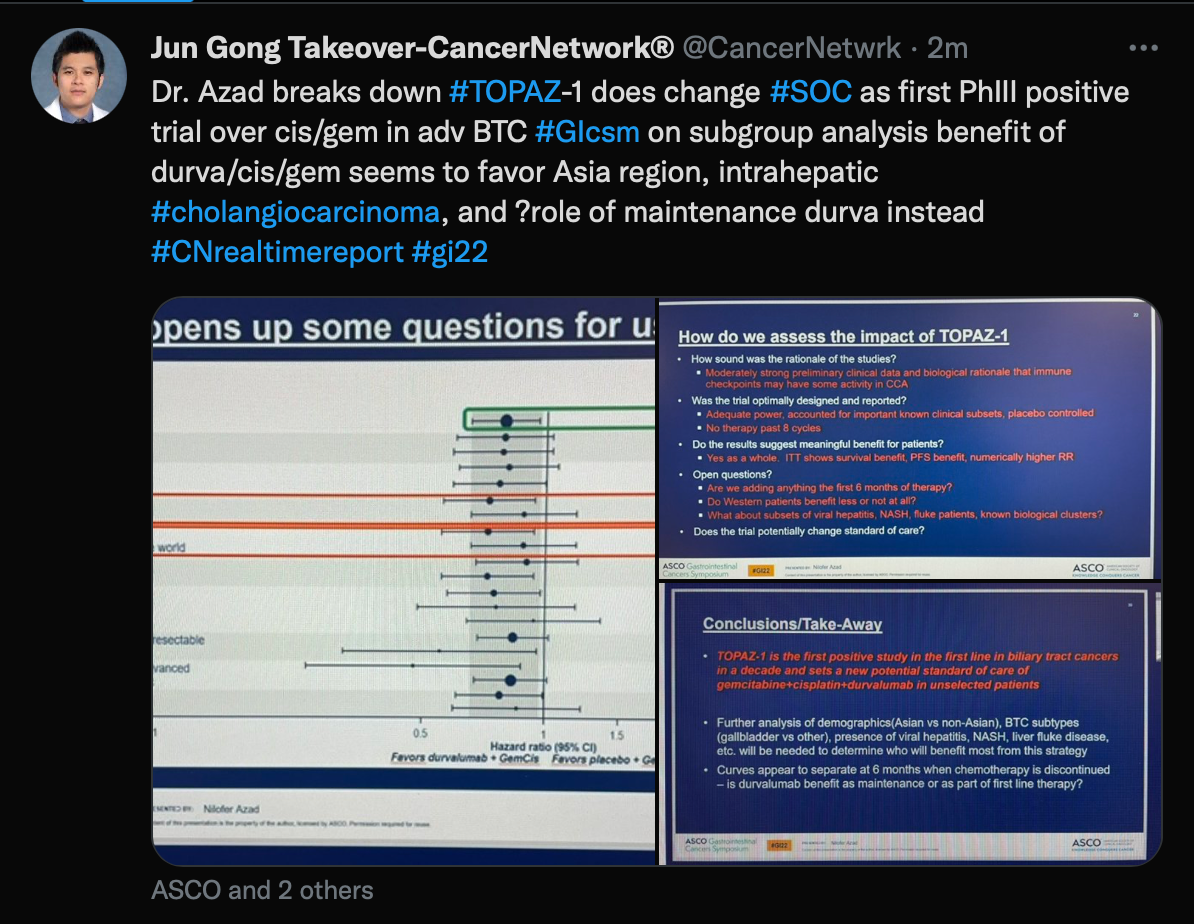
Next, Gong looked at data from a discussion of the phase 3 TOPAZ-1 trial (NCT08375235) in patients with biliary tract cancers. This discussion was led by Nilofer Saba Azad, MD, a professor of oncology at Johns Hopkins University School of Medicine. She spoke about the positive impact this trial made on the standard of care in patients with biliary tract cancer.
Gong wrote, “Dr. Azad breaks down #TOPAZ-1 does change #SOC as first PhIII positive trial over cis/gem in adv BTC…”
The trial investigated the use of durvalumab (Imfinzi) plus gemcitabine and cisplatin compared with a placebo and chemotherapy. At median follow-up times of 13.7 months and 12.6 months in the durvalumab and placebo arms, respectively, it was found that the addition of durvalumab led to better OS at 12.8 months (95% CI, 11.1-14.0) vs 11.5 months (95% CI, 10.1-12.5) with placebo. The 18-month OS rate for the durvalumab group was 35.1% (95% CI, 29.1%-41.2%) vs 25.6% (955 CI, 19.9%-31.7%) in the placebo arm; 24-month OS rates were 24.9% (95% CI, 17.9%-32.5%) and 10.4% (95% CI, 4.7%-18.8%), respectively.
KEYNOTE-590
Tweet of the KEYNOTE-590 trial

Finally, Gong spoke about the KEYNOTE-590 trial (NCT03189719) of first-line pembrolizumab (Keytruda) plus chemotherapy for patients with esophageal cancer or gastroesophageal junction adenocarcinoma, with a 12-month subgroup analysis follow-up showing a clinical benefit with the experimental regimen.
The median OS was 12.4 months in the pembrolizumab group vs 9.8 months in the chemotherapy alone group (HR, 0.73; 95% CI, 0.63-0.86). Additionally, the 1-year OS rates were 51% and 39%, respectively, with 2-year OS rates of 26% and 16%.
In the overall population, the PFS was 6.3 months in the pembrolizumab group and 5.8 months in the chemotherapy alone group (HR, 0.64; 95% CI, 0.55-0.75). The 1-year PFS rates were 25% and 12%, respectively, with 2-year PFS rates of 12% and 3%.
Gong concluded with, “…no new safety signals, no deterioration in QoL, 20% vs 6% response duration >24 mos.”
To view the full Twitter takeover, search #CNRealtimeReport on Twitter