CancerNetwork® Recaps Gong’s Twitter Takeover at ASCO GU 2022
Jun Gong, MD, hosted a Twitter takeover during the 2022 Genitourinary Cancers Symposium where he discussed breaking presentations in a #CNRealtimeReport.
Jun Gong, MD

The 2022 Genitourinary Cancers Symposium hosted by the American Society of Clinical Oncology (ASCO GU) and other industry cosponsors brought top thought leaders together to present breaking data in areas such as prostate cancer, urothelial cancer, and renal cell carcinoma.
CancerNetwork® hosted Jun Gong, MD, a medical oncologist of the Gastrointestinal Disease Research Group, Pancreatic Research Group, and Urologic Oncology Program at the Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai in Los Angeles, California, as moderator for its Twitter takeover for the entirety of the conference. Gong covered multiple oral abstract sessions and broke down the presentations in a #CNRealtimeReport.
ARASENS Trial
Tweet of the ARASENS trial
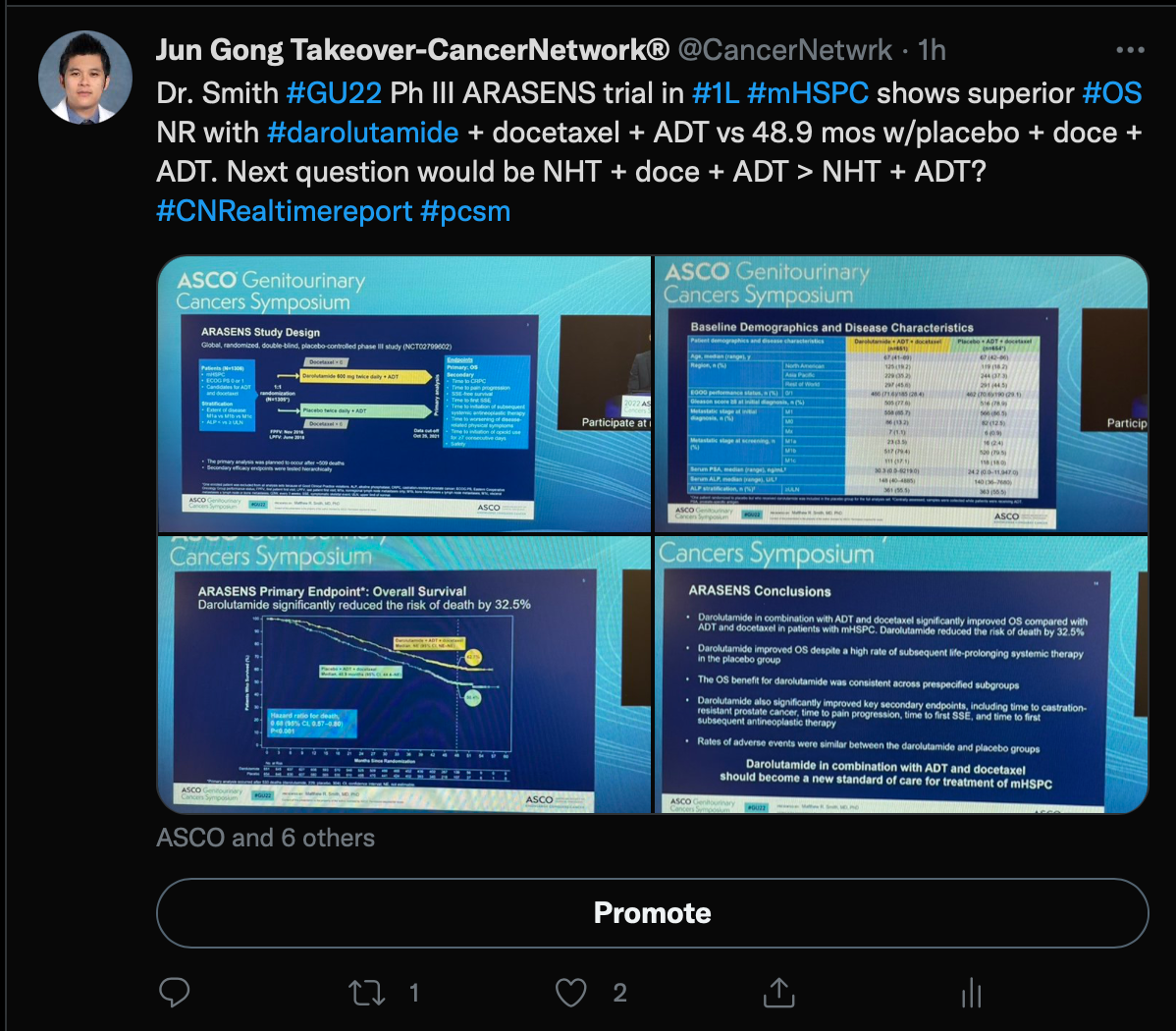
Gong began the takeover by discussing the ARASENS trial (NCT02799602), which included men with metastatic castration-sensitive prostate cancer who were treated with androgen deprivation therapy (ADT) and docetaxel with or without darolautamide (Nubequa).
At a follow-up of 43.7 months for the darolutamide group, a 32.5% reduction in the risk of death was seen in the darolatumaide group (HR, 0.68; 95% CI, 0.57-0.80; P <.001). The median overall survival was not estimable (NE) in the darolautamide group vs 48.9 months (95% CI, 44.4-NE) in the placebo group.
Additionally, time to castration-resistant disease was NE in the darolutamide group and 19.1 months (95% CI, 16.5-21.8) in the placebo group (HR, 0.36; 95% CI, 0.30-0.42; P <.001). Darolutamide also had a longer time to increased pain progression that was NE vs 27.5 months (95% CI, 22.0-36.1) in the placebo group (HR, 0.79; 95% CI, 0.66-0.95; P = .01).
Gong concluded by posing a question about whether the addition of docetaxel to a combination with novel hormonal therapy is necessary or if darolutamide plus ADT works just as well without the chemotherapeutic.
PRESIDE Trial
Tweet of the PRESIDE trial
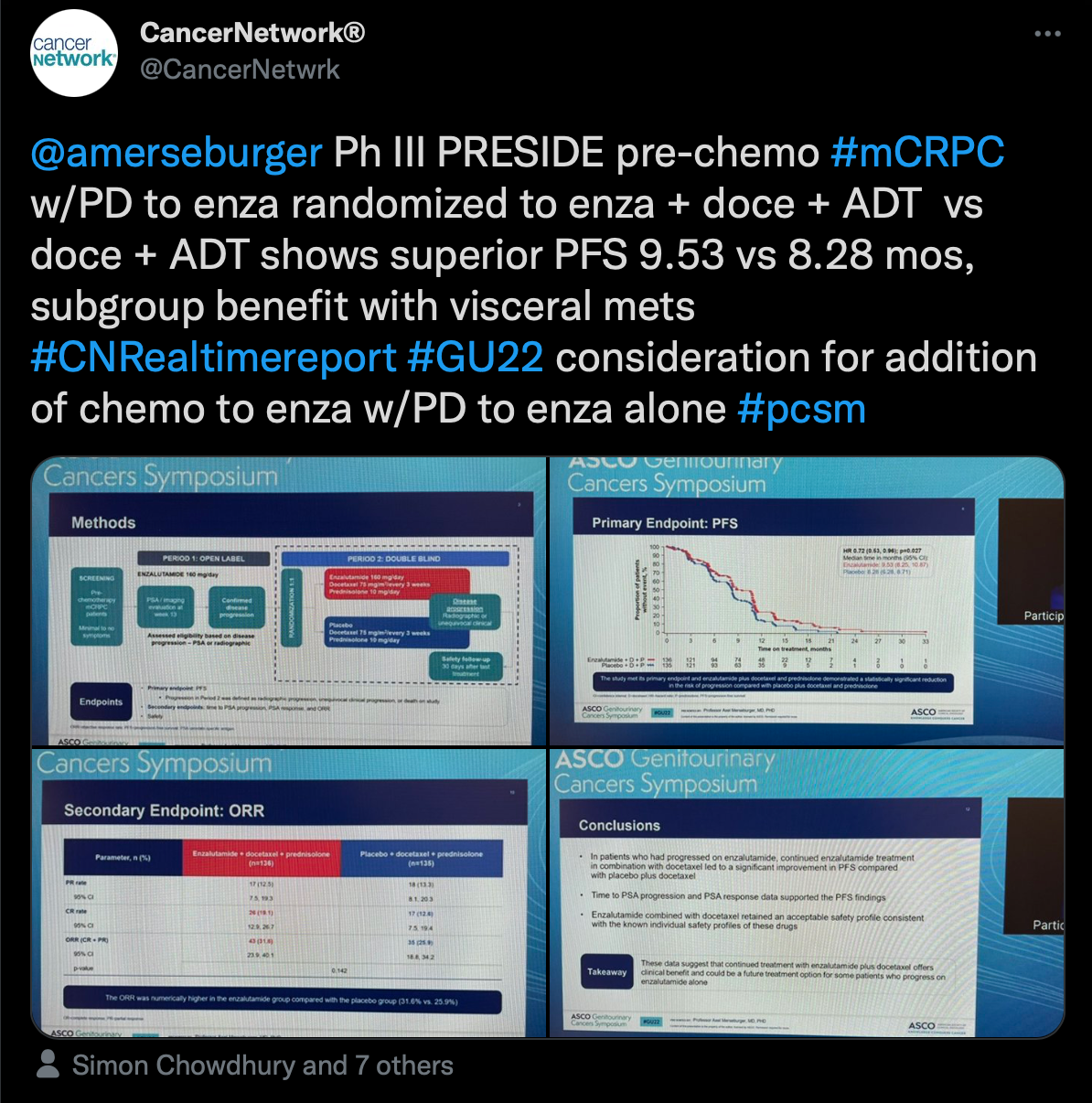
Another highlight from ASCO GU was the PRESIDE trial (NCT02288247) presented by Axel Merseburger, MD, chairman of the Clinic of Urology at University Hospital Schleswig-Holstein in Lübeck, Germany. Results of the trial showed that continuous enzalutamide (Xtandi) vs placebo, both plus docetaxel and prednisone, for patients with metastatic castration-resistant prostate cancer (CRPC) improved progression-free survival (PFS) outcomes for those who had previously progressed on the androgen receptor inhibitor alone.
“Ph III PRESIDE pre-chemo #mCRPC w/PD to enza randomized to enza + doce +ADT vs doce + ADT shows superior PFS,” Gong wrote.
Patients with metastatic castration-resistant prostate cancer had a median PFS of 9.53 months (95% CI, 8.25-10.87) in the enzalutamide group and 8.28 months (95% CI, 6.28-8.71) in the placebo group (HR, 0.72; 95% CI, 0.53-0.96; P = .027). Additionally, patients were more likely to experience a decrease in their prostate-specific antigen from baseline to week 13 at –37.12% in the enzalutamide group and 9.11% in the placebo group.
The safety profile remained consistent with what was previously noted for enzalutamide.
EV-103 Study
Tweet of the EV-103 study
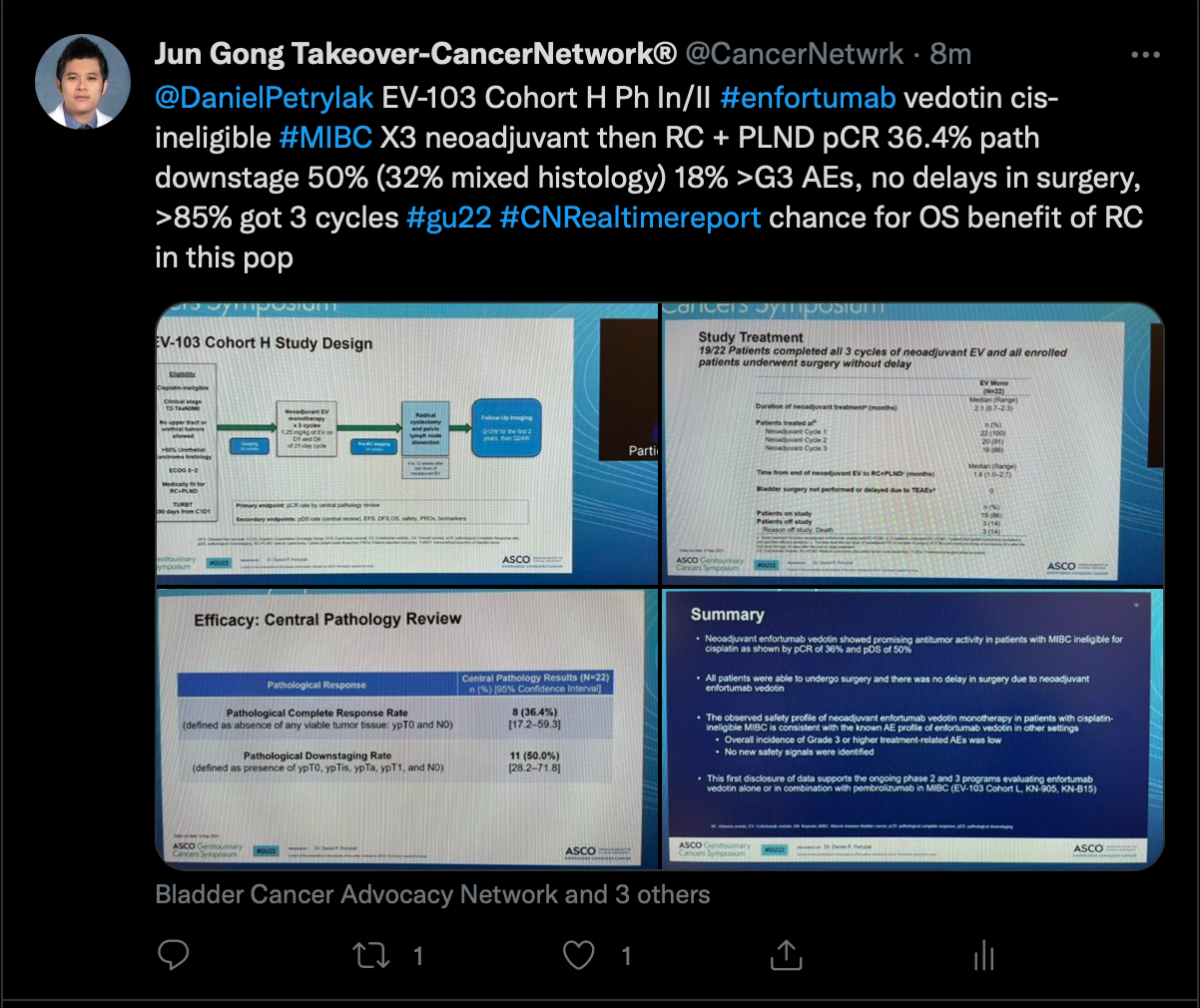
Next, Gong discussed a presentation from the phase 1b/2 EV-103 study (NCT02388545) which examined data from a cohort of patients with muscle-invasive bladder cancer who were treated with neoadjuvant enfortumab vedotin (Padcev) in the setting of cisplatin ineligibility.
A pathological complete response was observed in 36.4% (95% CI, 17.2%-59.3%). Moreover, pathological downstaging was seen in 50.0% of patients (95% CI, 28.2%-71.8%).
Grade 3 treatment-related adverse effects included asthenia, dehydration, erythema multiforme, and hyperglycemia. There were no grade 4 events.
KEYNOTE-564
Tweet of the KEYNOTE-564 trial
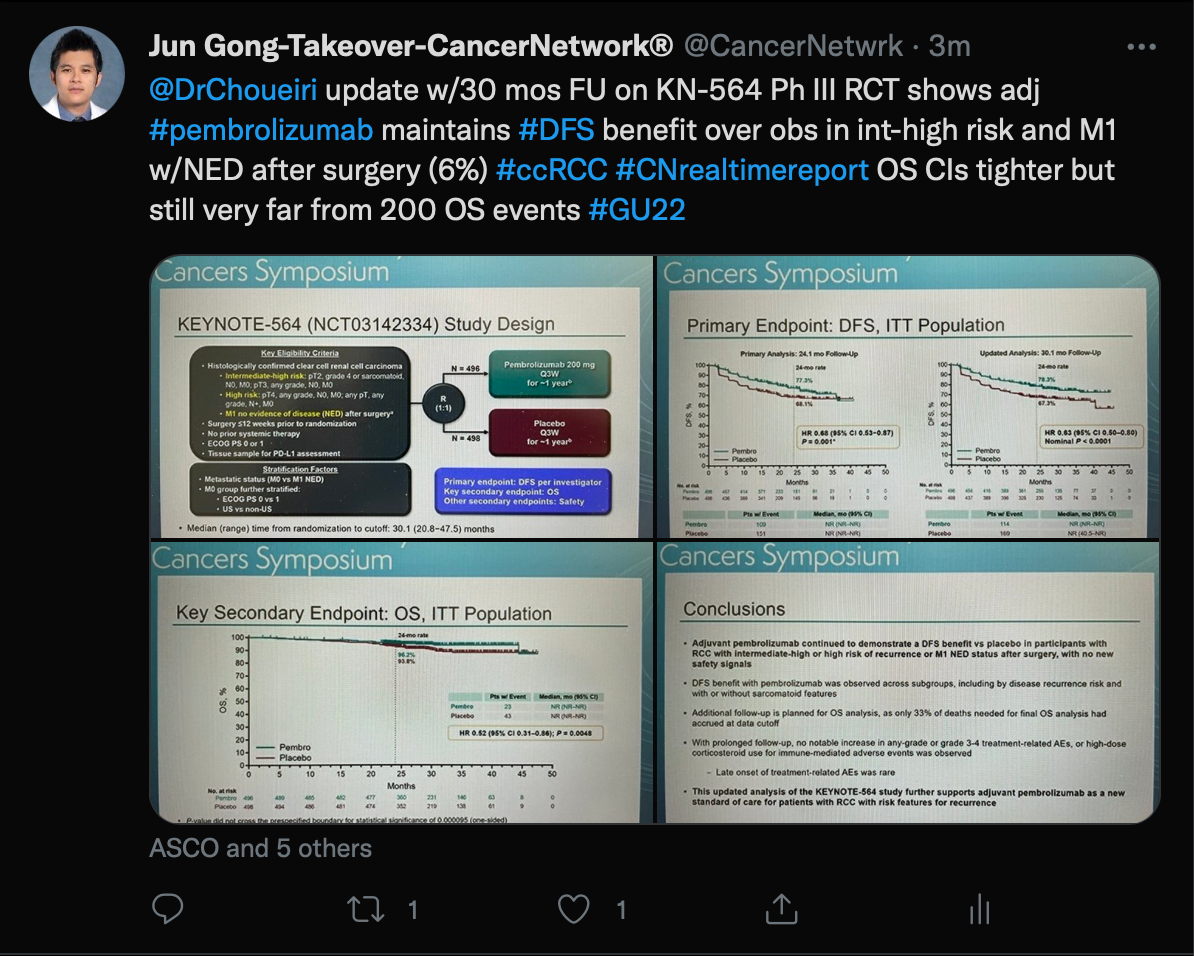
Finally, Gong spoke about an updated analysis of the KEYNOTE-564 trial (NCT03142334) which was presented by Toni K. Choueiri, MD, director of the Lank Center for Genitourinary Oncology at Dana-Farber Cancer Institute and Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School in Boston, Massachusetts.
This trial examined patients with renal cell carcinoma receiving pembrolizumab (Keytruda) as adjuvant therapy following nephrectomy vs a placebo. The disease-free survival (DFS) benefit was maintained in the overall pembrolizumab group (HR, 0.63; 95% CI, 0.50-0.80; nominal P <.0001) as well as additional subgroups of patients with intermediate-high risk (HR, 0.68; 95% CI, 0.52-0.89) and high-risk (HR, 0.60; 95% CI, 0.33-1.10) disease plus in those with M1 no evidence of disease (HR, 0.28; 95% CI, 0.12-0.66).
Previous analyses found this combination resulted in a statistically significant DFS improvement over the placebo in the intent-to-treat population (HR, 0.68; 95% CI, 0.53-0.87; P = .001).
To view the full Twitter takeover, search #CNRealtimeReport on Twitter.