Carboplatin Therapy Intensification Results in Improved 5-Year EFS in Pediatric High-Risk Group 3 Medulloblastoma
Pediatric patients with high-risk molecular subgroup group 3 medulloblastoma experienced an improvement in 5-year event-free survival after receiving therapy intensification with carboplatin.
The use of therapy intensification with carboplatin worked to improve 5-year event-free survival (EFS) in a population of pediatric patients with high-risk group 3 medulloblastoma, according to the results of a phase 3 study (NCT00392327) published in JAMA Oncology.
Investigators reported that the use of therapy intensification with carboplatin yielded a 19% higher survival in pediatric patients with group 3 medulloblastoma. The 5-year EFS rate was 66.4% (95% CI, 56.4%-76.4%) with carboplatin and 59.2% (95% CI, 48.8%-69.6%; P = .11) in the control arm. In patients who were specifically in the group 3 subgroup, the 5-year EFS rate was 73.2% (95% CI, 56.9%-89.5%) with carboplatin compared with 53.7% (95% CI, 35.3%-72.1%) without (P = .047).
“In this prospective, phase 3 randomized clinical trial, we did not observe a sufficient benefit to recommend either carboplatin or isotretinoin for all children with high-risk medulloblastoma. Although the study was initially powered to evaluate medulloblastoma as a single disease, biologic insights have led to a contemporary view of medulloblastoma as 4 molecularly distinct subgroups that may determine response to therapy and prognosis,” investigators of the study wrote.
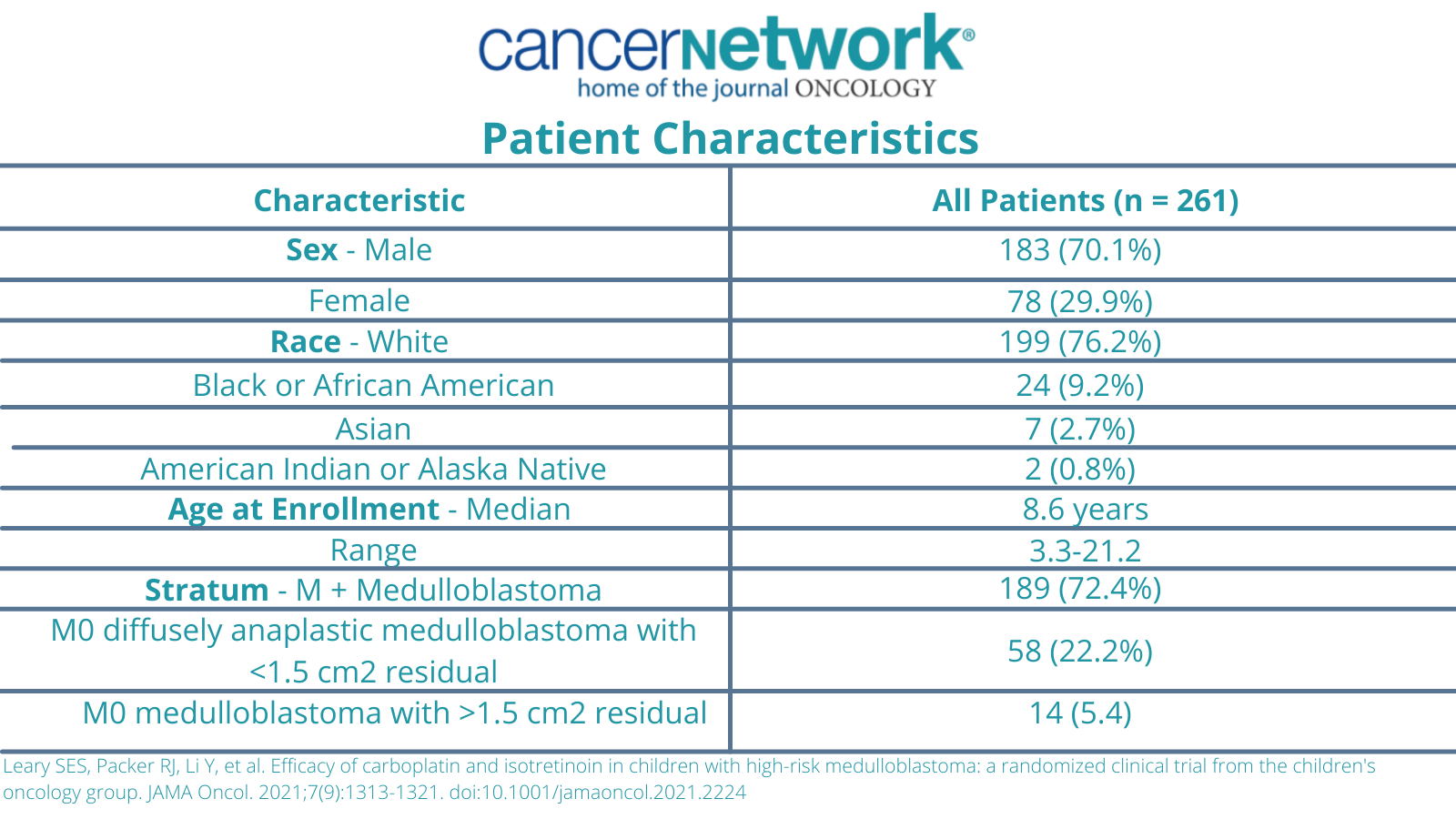
The study initially enrolled 294 patients, 9 of whom were ineligible due to the timing of the therapy, as well as an additional 24 patients who were ineligible due to retrospective central or central pathologic review. The median follow-up time was 6.7 years. A total of 15 patients were lost to follow up before 5-years. Additionally, 261 patients were eligible by both prospective and retrospective central review.
At the time of the enrollment, the median patient age was 8.6 years (range, 3.3-21.2). A total of 72.4% (n = 189) of patients had metastatic disease and 22.2% (n = 58) had diffuse anaplasia. Additionally, 5.4% (n = 14) of patients had residual disease of 1.5 cm2 or greater.
Patient Characteristics of a phase 3 study with carboplatin intensification in Pediatric Medulloblastoma
Patients were randomized to receive 36 Gy of craniospinal radiation therapy plus weekly vincristine plus or minus daily carboplatin. This was followed by 6 cycles of maintenance chemotherapy with cisplatin, cyclophosphamide, and vincristine plus or minus 12 cycles of treatment with isotretinoin (Absorica) and maintenance therapy.
Investigators discontinued the use of isotretinoin as investigators believed it was unlikely to lead to a significant EFS difference. The 5-year EFS with the use of isotretinoin was 68.6% (95% CI, 52.1%-85.1%) vs without isotretinoin was 67.8% (95% CI, 47.6%-88.0%).
Among patients in the group 3 cohort who had M0 stage disease (n = 20; 19 with anaplasia, 1 with residual), the 5-year EFS rate was 95.0% (95% CI, 84.2%-100%) and the overall survival (OS) was 100% (95% CI, 100%-100%). Comparatively, patients who had metastatic disease achieved a 5-year EFS rate of 53.6% (95% CI, 37.9%-69.3%) and OS 64.9% (95% CI, 50.2%-79.6%).
Patients who had metastatic disease and group 3 medulloblastoma, the 5-year EFS with carboplatin was 64.8% (95% CI, 43.8%-85.8%) and the OS was 77.4% (95% CI, 58.8%-96.0%). Comparatively, the 5-year EFS for without carboplatin was 40.3% (95% CI, 19.9%-60.7%; P = 0.45) and the OS was 51.2% (95% CI, 31.0%-71.4%; P = .046).
Additionally, an association was identified between MYC amplification or isochromosome 17 and inferior survival in patients with group 3 medulloblastoma. This translated to a 5-year EFS rate was 49.3% (95% CI, 29.5%-69.1%) vs 73.6% among those who did not have a MYC amplification or isochromosome 17 (95% CI, 56.9%-90.3%; P = .01).
In terms of safety, hematologic adverse effects (AEs) appeared to be more notable in patients who were treated with carboplatin during the induction phase. This resulted in an increased risk of thrombocytopenia and febrile neutropenia. High grade toxicities of grade 3 or higher were observed in more than 5% of the study’s patients. Grade 3 anaphylaxis to carboplatin was reported in 4 patients in the carboplatin arm.
Investigators also assessed 5-year EFS across different molecular subgroups who received treatment with carboplatin, including WNT (92.9%; 95% CI, 75.7%-100%), SHH (49.6%; 95% CI, 27.8%-71.4%), and group 4 65.6% (95% CI, 54.6%-76.6%).
“We observed 19% higher survival exclusively in group 3 medulloblastoma patients who were randomized to receive intensified chemoradiotherapy with concurrent carboplatin, and 25% higher survival for metastatic group 3 medulloblastoma patients. However, improved survival comes at a cost of increased toxic effects. It is therefore appropriate to avoid therapy intensification in groups of patients who would not be expected to benefit, specifically in those with WNT, SHH, and group 4 medulloblastoma,” the study’s investigators concluded.
Reference:
Leary SES, Packer RJ, Li Y, et al. Efficacy of carboplatin and isotretinoin in children with high-risk medulloblastoma: A randomized clinical trial from the Children's Oncology Group. JAMA Oncology. Published Online July 22, 2021. doi:10.1001/jamaoncol.2021.2224