Carboplatin/TP More Effective Than TP in HER-2+ Breast Cancer
SAN ANTONIO-The three-drug combination of carboplatin (Paraplatin), paclitaxel (Taxol), and trastuzumab (Herceptin) increases response rate and time to progression among HER-2-positive patients with advanced breast cancer, compared with the trastuzumab/paclitaxel combination alone, according to Nicholas J. Robert, MD, chairman of research at Inova Fairfax Hospital’s Cancer Center, Fairfax, Virginia, and co-chairman of the Breast Committee of the US Oncology Research Network.
SAN ANTONIOThe three-drug combination of carboplatin (Paraplatin), paclitaxel (Taxol), and trastuzumab (Herceptin) increases response rate and time to progression among HER-2-positive patients with advanced breast cancer, compared with the trastuzumab/paclitaxel combination alone, according to Nicholas J. Robert, MD, chairman of research at Inova Fairfax Hospital’s Cancer Center, Fairfax, Virginia, and co-chairman of the Breast Committee of the US Oncology Research Network.
Speaking at the 25th Annual San Antonio Breast Cancer Symposium (abstract 35), Dr. Robert noted that the improvements in efficacy were accompanied by moderate, but acceptable, increases in toxicity.
As background, Dr. Robert reviewed the results of the pivotal trial that established the superiority of trastuzumab and paclitaxel (TP) over paclitaxel alone. In that trial, the TP combination produced a response rate of 41% vs 17% for pac-litaxel alone. Time to progression increased from 3.0 months on the single-agent therapy to 6.9 months on the combination.
Looking for a regimen to improve upon these results, the researchers considered the documented activity of carboplatin and taxanes. In three phase II trials of first-line paclitaxel and carboplatin in breast cancer patients, Dr. Robert noted, the combination produced response rates ranging from 53% to 62%.
"These response rates are competitive with those of anthracycline-containing regimens, and we know that anthracy-cline-containing regimens are active with trastuzumab," he said. "But we’re also concerned about the increased cardiotox-icity of greater than 25%. With this approach, we hoped to find a regimen where we could avoid the anthracycline/trastuzumab cardiotoxicity."
The current phase III trial was designed to evaluate response rate, time to progression, survival, and toxicity among stage IV, HER-2-positive breast cancer patients receiving trastuzumab, pacli-taxel, and carboplatin (TPC) vs TP alone.
The trial enrolled 196 patients who were designated HER-2 2+ or 3+ by immunohistochemistry (IHC) (Hercep-Test) in a central laboratory. The trial was later amended so that 2+ patients had to be positive on fluorescent in situ hybridization (FISH) to be eligible. Patients had received no prior chemotherapy for metastatic disease and had an ECOG performance status of 0 to 2.
Exclusion criteria were current chemotherapy or hormonal therapy, abnormal left ventricular ejection fraction, prior adjuvant taxanes, or a cumulative lifetime dose of doxorubicin of greater than 360 mg/m2.
The control arm of the study was similar to the investigative arm of the pivotal trial. Trastuzumab was administered at a loading dose of 4 mg/kg, followed by weekly doses of 2 mg/kg. Paclitaxel was administered at 175 mg/m2 over 3 hours every 21 days. Patients had to be treated for at least six cycles and, at the investigator’s discretion, could continue treatment after that. The treatment group received the same regimen of paclitaxel and trastuzumab but with carboplatin at AUC 6 every 21 days.
Patient characteristics were well balanced in both arms. The median age was 55 (range, 32 to 82), and 40% of the patients had received adjuvant chemotherapy. The majority of patients had visceral-dominant disease, and more than half had metastatic disease at more than one site. The two groups were comparable in terms of estrogen-receptor status and had a nearly identical distribution of IHC 2+, 3+ status.
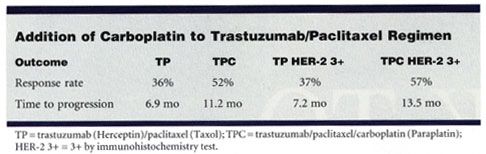
The objective response rate was 52% with TPC, compared with 36% with TP (P = .04), Dr. Robert reported. In a subset analysis based on IHC status, the response rate of 3+ patients on TPC was 57% vs 37% for those taking TP (P = .03). Among the smaller population of FISH-positive patients, a similar trend was noted59% of TPC patients responded vs 42% of TP patients.
The three-drug regimen also proved superior in terms of time to progression11.2 months among patients given TPC vs 6.9 months among those given TP (P = .007). Time to progression among 3+ patients on TPC was nearly double that of those on TP13.5 months vs 7.2 months (P = .006).
The TPC regimen offered similar benefits in progression-free survival: At 12 months, 45% of the TPC patients were progression free, compared with 33% of the TP group. At 24 months, the progression-free survival was 25% for TPC vs 11% for TP, and at 36 months, it was 18% vs 8%.
"When we look at our trial compared to the pivotal trial, there has been a step-wise improvement in the care of patients with HER-2-positive disease," Dr. Robert said. "When you add trastuzumab to paclitaxel, you see an improvement in response rate from 17% to 41%, and when you add carboplatin to this combination the response rate increases to 52%."
In terms of overall survival, Dr. Robert said that it is too early for a final analysis because more than 100 patients are still alive, but a preliminary estimate is possible.
The trend for overall survival at 12 months is 92% for TPC, compared with 87% for TP (
P =
.02). At 24 months, the overall survival trend for TPC compared with TP is 75% vs 66%, and at 36 months, it is 62% vs 47%.
As anticipated, the addition of carboplatin to the TP regimen resulted in more myelosuppression. The incidence of neutropenia in the TPC group was 85.6%, compared with 24.2% in the TP group; leukopenia, 11.3% (TPC) vs 6.3% (TP); and thrombocytopenia, 9.3% (TPC) vs 1.1% (TP). None of these increases, however, translated into increased infections, fever, or bleeding in the TPC group.
The occurrence of other side effects, such as anemia, fatigue, neuropathy, nausea, arthralgia, and allergic reactions, was similar in the two treatment groups. In terms of left-ventricular dysfunction, one patient in the TP arm developed congestive heart failure.
"Based on the study findings, physicians should consider adding carboplatin to the standard combination regimen of paclitaxel and trastuzumab in eligible patients with advanced HER-2-positive breast cancer," Dr. Robert said. "With these encouraging findings, we will now study this combination therapy in earlier stages of breast cancer."