The cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors palbociclib, ribociclib, and abemaciclib are rapidly transforming the care of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (HR+/HER2−) advanced breast cancer. Current clinical questions include how to choose among these agents and how to sequence them with other therapies. Areas of active inquiry include identifying predictive biomarkers for CDK4/6 inhibitors, deciding whether to continue CDK4/6 inhibitors after disease progression, creating novel treatment combinations, and expanding use beyond HR+/HER2− advanced breast cancer. Here, we review the current use of and potential next directions for CDK4/6 inhibitors in the treatment of patients with HR+ breast cancer.
Introduction
Despite the availability of many therapies, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (HR+/HER2−) advanced breast cancer is rarely curable. The current treatment paradigm for HR+/HER2− advanced breast cancer involves sequencing endocrine therapy, targeted therapy, and/or chemotherapy to prolong patients’ lives, delay disease progression, and minimize cancer-related symptoms. The cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors are rapidly transforming this treatment landscape. There are currently three CDK4/6 inhibitors that have been approved by the US Food and Drug Administration: palbociclib, ribociclib, and abemaciclib. How to choose among these agents and how to sequence them with other therapies are currently the most pressing questions. The possibility of using biomarkers to predict response, novel treatment combinations with CDK4/6 inhibitors, and the potential activity of these agents beyond the setting of HR+/HER2− advanced breast cancer are areas of active research. We will review the current role of CDK4/6 inhibitors in the treatment of patients with HR+ breast cancer, as well as promising future applications.
Mechanism of Action of CDK4/6 Inhibitors
The CDK4/6 inhibitors act at the G1-to-S cell cycle checkpoint. This checkpoint is tightly controlled by the D-type cyclins and CDK4 and CDK6. When CDK4 and CDK6 are activated by D-type cyclins, they phosphorylate the retinoblastoma-associated protein (pRb). This suspends pRb’s suppression of the E2F transcription factor family and ultimately allows the cell to proceed through the cell cycle and divide. In HR+ breast cancer, cyclin D overexpression is common and loss of pRb is rare, making the G1-to-S checkpoint an ideal therapeutic target. The CDK4/6 inhibitors prevent progression through this checkpoint, leading to cell cycle arrest.[1]
Approved CDK4/6 Inhibitors for HR+/HER2− Advanced Breast Cancer
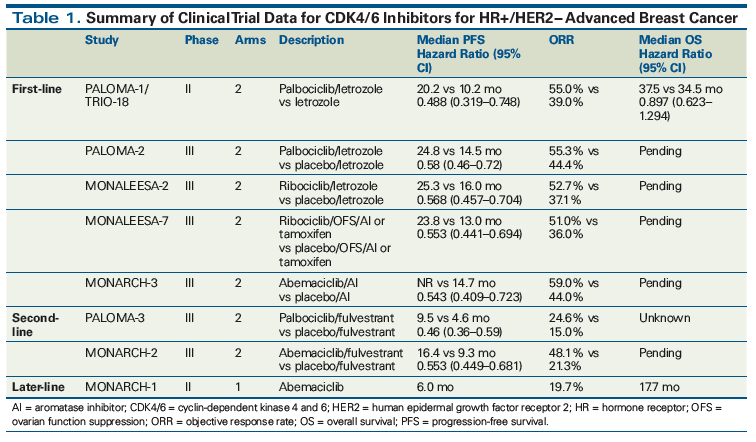
Table 1 summarizes the evidence from the pivotal trials that led to the FDA approveals of palbociclib, ribociclib, and abemaciclib.
Palbociclib
Palbociclib was approved for use with an aromatase inhibitor (AI) as first-line treatment of HR+/HER2− advanced breast cancer in postmenopausal women, based on the phase II study PALOMA-1/TRIO-18 and the phase III study PALOMA-2. Palbociclib was approved for use with fulvestrant as second- or later-line treatment of HR+/HER2− advanced breast cancer based on the phase III study PALOMA-3.
In PALOMA-1/TRIO-18, 165 women with HR+/HER2− untreated advanced breast cancer were randomized to receive either palbociclib and letrozole or letrozole alone. Previous treatment with an AI more than 12 months before enrollment was allowed. The study’s primary endpoint was median progression-free survival (PFS). Patients who received palbociclib and letrozole had a median PFS of 20.2 months, compared with only 10.2 months with letrozole alone (hazard ratio, 0.488; 95% CI, 0.319–0.748; P = .0004).[2] This study led to accelerated approval of palbociclib in February 2015. Median overall survival (OS) was 37.5 months with palbociclib and letrozole and 34.5 months with letrozole alone (HR, 0.897; 95% CI, 0.623–1.294; P = .281). This difference was not significant, but the study was not powered to show a difference in OS.[3]
In PALOMA-2, 666 treatment-naive patients with HR+/HER2− advanced breast cancer were randomized to receive palbociclib and letrozole or placebo and letrozole. Median PFS was 24.8 months in the patients who received palbociclib and letrozole and 14.5 months in those who received placebo and letrozole (hazard ratio, 0.58; 95% CI, 0.46–0.72; P = .001).[4] This led to regular approval of palbociclib in March 2017. OS data are still maturing.
In PALOMA-3, 521 women of any menopausal status with HR+/HER2− advanced breast cancer whose disease had progressed on prior endocrine therapy or recurred within 12 months of stopping adjuvant endocrine therapy were randomized to receive either palbociclib and fulvestrant or placebo and fulvestrant. Approximately half the patients had received two or more lines of endocrine therapy in the metastatic setting and approximately one-third had received chemotherapy in the metastatic setting. Final analysis demonstrated a median PFS in the palbociclib and fulvestrant group of 9.5 months, compared with 4.6 months in the placebo and fulvestrant group (hazard ratio, 0.46; 95% CI, 0.36–0.59; P < .0001),[5,6] which led to the approval of palbociclib in combination with fulvestrant for use after progression while receiving endocrine therapy.
Ribociclib
Ribociclib was approved in March 2017 for first-line treatment of HR+/HER2− advanced breast cancer in postmenopausal women, based on the results of the phase III MONALEESA-2 study. In this study, treatment-naive patients with HR+/HER2− advanced breast cancer received letrozole with ribociclib or placebo. Prior AI therapy was allowed if it had been discontinued 12 months before enrollment. At the 18-month follow-up, median PFS had not been reached in the ribociclib-treated arm, compared with a median PFS of 14.7 months in the placebo group (hazard ratio, 0.56; 95% CI, 0.43–0.72; P < .001). Updated analysis showed a median PFS of 25.3 months in the ribociclib group vs 16.0 months in the placebo group.[7,8] OS data are not available yet.
MONALEESA-3 is a phase III study assessing ribociclib in combination with fulvestrant for both second-line and first-line treatment of HR+/HER2− advanced breast cancer in both postmenopausal women and men. This study has completed recruitment but results are not yet available.
Abemaciclib
Abemaciclib was approved in February 2018 in combination with an AI for first-line therapy of HR+/HER2− advanced breast cancer in postmenopausal women, based on results from the MONARCH-3 study. Abemaciclib was approved in September 2017 for second- or later-line therapy in combination with fulvestrant, based on the results of MONARCH-2, and as a single agent for third- or later-line therapy for women and men, based on MONARCH-1.
MONARCH-3, a phase III study, compared an AI with abemaciclib or placebo in treatment-naive patients with HR+/HER2− advanced breast cancer. Interim results after 18 months of follow-up demonstrated that median PFS was not reached in the abemaciclib arm, compared with a median PFS of 14.7 months in the placebo arm (hazard ratio, 0.543; 95% CI, 0.409–0.723; P < .001).[9,10]
MONARCH-2 was a phase III study that randomized patients with HR+/HER2− advanced breast cancer whose disease had progressed on prior endocrine therapy to receive either abemaciclib and fulvestrant or placebo and fulvestrant. Patients could enroll if their disease had progressed during adjuvant or neoadjuvant endocrine therapy, ≤ 12 months after completion of adjuvant endocrine therapy, or while receiving endocrine therapy for advanced breast cancer. Patients had received only one prior line of endocrine therapy and no chemotherapy for their advanced breast cancer. Median PFS in the abemaciclib group was significantly improved: 16.4 months, compared with 9.3 months in the placebo group (hazard ratio, 0.553; 95% CI, 0.449–0.681; P < .001).[11] OS data are still maturing.
MONARCH-1 was a phase II single-arm study of abemaciclib as a single agent; it enrolled women of any menopausal status with HR+/HER2− advanced breast cancer that had progressed on prior endocrine therapy and chemotherapy (at least one line of chemotherapy). Patients in this study had received a median of three prior lines of systemic treatment for advanced breast cancer. The majority (90.2%) had visceral disease. The objective response rate (ORR) was 19.7% at 12 months, median PFS was 6.0 months, and median OS was 17.7 months.[12]
Comparative Efficacy of CDK4/6 Inhibitors for HR+/HER2− Advanced Breast Cancer
Palbociclib, ribociclib, and abemaciclib are approved in combination with an AI for first-line therapy of HR+/HER2− advanced breast cancer. These drugs have never been directly compared and are considered equivalent in efficacy. Palbociclib and ribociclib demonstrated similar prolongations of PFS when compared with AI-only therapy in PALOMA-1/PALOMA-2 and MONALEESA-2, respectively. Data regarding abemaciclib with an AI vs an AI only are comparatively immature. Only results from 18 months of follow-up have been reported thus far; however, the hazard ratio is similar to that seen in studies with palbociclib and ribociclib, suggesting similar efficacy. All three agents are appropriate choices in combination with an AI for first-line treatment.
Palbociclib and abemaciclib are both approved in combination with fulvestrant for second- or later-line treatment of HR+/HER2− advanced breast cancer, and either combination is an appropriate choice in this setting. There are no direct comparisons of these two agents and they are likely equivalent in efficacy. Of note, the patients who received abemaciclib-fulvestrant in MONARCH-2 had a much longer PFS (16.4 months) than did patients who received palbociclib-fulvestrant in PALOMA-3 (9.5 months). This likely reflects differences in the characteristics of the patient populations enrolled. The patients in MONARCH-2 had received only one prior line of endocrine therapy, and 59% had received only adjuvant or neoadjuvant endocrine therapy. No patients had received chemotherapy in the metastatic setting. In contrast, about half the patients enrolled in PALOMA-3 had received two or more lines of endocrine therapy in the metastatic setting, and about one-third had received chemotherapy in the metastatic setting, indicating a population that was more endocrine-resistant and possibly more treatment-resistant. Consequently, the median PFS for patients in the control (fulvestrant alone) arm of MONARCH-2 was much longer than for patients in the control arm of PALOMA-3 (9.3 vs 4.6 months). Ribociclib with fulvestrant will likely have activity similar to that of the palbociclib-fulvestrant and abemaciclib-fulvestrant combinations.
CDK4/6 Inhibitors in Premenopausal Women
The combination of palbociclib or ribociclib with an AI is only approved for postmenopausal women. Many providers are treating premenopausal women with a CDK4/6 inhibitor, an AI, and ovarian function suppression with a gonadotropin-releasing hormone agonist, based on studies showing that an AI with ovarian suppression is efficacious for premenopausal women with HR+/HER2− advanced breast cancer.[13] Several ongoing studies specifically include premenopausal women receiving palbociclib or ribociclib and endocrine therapy with ovarian function suppression (eg, FATIMA [ClinicalTrials.gov identifier: NCT02917005], COMPLEEMENT-1 [NCT02941926]).
MONALEESA-7 was the first of these trials that included premenopausal women to be reported. In this phase III study, premenopausal women with HR+/HER2− advanced breast cancer were treated with first-line ribociclib vs placebo, along with goserelin and tamoxifen or a nonsteroidal AI. The improvement in PFS seen in the ribociclib arm (23.8 vs 13.0 months [hazard ratio, 0.553; 95% CI, 0.441–0.694; P < .001]) was similar to that seen in postmenopausal women in MONALEESA-2.[14]
In PALOMA-3, premenopausal women were included and received goserelin. A subgroup analysis of the PALOMA-3 study showed that premenopausal women derived a benefit similar to that seen in the overall study population.[15] The combination of palbociclib and fulvestrant is approved regardless of menopausal status.
None of the first-line studies looked at the activity of CDK4/6 inhibitors in premenopausal women in the absence of ovarian suppression. In MONARCH-1, a single-arm study of abemaciclib as a single agent, women were enrolled regardless of their menopausal status. However, all had demonstrated prior progression on at least one line of chemotherapy for advanced disease, so presumably the premenopausal women had some degree of ovarian failure while enrolled in the study.
Safety of CDK4/6 Inhibitors
The CDK4/6 inhibitors as a class are generally well tolerated. The most common class-wide adverse effects include nausea, diarrhea, fatigue, neutropenia, leukopenia, anemia, and thrombocytopenia. Palbociclib and ribociclib most commonly cause neutropenia, while diarrhea is the most common adverse effect of abemaciclib, perhaps because of its greater affinity for CDK4 over CDK6.[16] The safety profiles of these agents do not absolutely favor one agent over another, and patient-specific concerns related to toxicity should be taken into account. If a patient is intolerant of one agent, it is reasonable to try another.
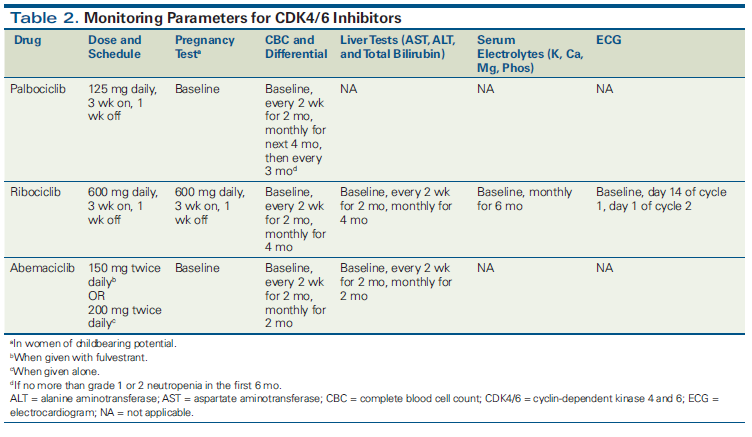
Palbociclib, administered at a dosage of 125 mg daily for 3 weeks on and 1 week off, was associated with grade 3/4 neutropenia in 55% to 65% of patients across clinical studies. Febrile neutropenia occurred in only 0% to 2% of patients, and permanent discontinuation of therapy was infrequent.[4,17] Complete blood count (CBC) should be monitored frequently early in therapy, and neutropenia should be managed with appropriate dose interruption and/or dose reduction. Palbociclib-induced neutropenia occurs via a unique mechanism, in which cell cycle arrest occurs without apoptosis of hematopoietic precursor cells.[18] This unique mechanism allows rapid reversal with dose interruption and/or dose reduction, unlike with chemotherapy-induced neutropenia. Detailed safety analysis of PALOMA-3 showed that neutropenia occurred early (median time to onset, 16 days), was reversible (median duration, 7 days), and was not cumulative (became increasingly rare with subsequent cycles). Dose reduction did not seem to impact efficacy.[17] Of note, grade 3 anemia, although uncommon, can be a late event, justifying continued hematologic monitoring.[19]
Ribociclib, at a dosage of 600 mg daily for 3 weeks on and 1 week off, was associated with grade 3/4 neutropenia in ~60% of patients in MONALEESA-2; however, febrile neutropenia was rare, as was permanent discontinuation of the study drug. Other important adverse effects that require monitoring include elevated alanine aminotransferase or aspartate aminotransferase levels and prolonged QT interval. CBC should be monitored frequently early in treatment, and neutropenia can be managed in a manner similar to that used for palbociclib-associated neutropenia.
Abemaciclib, at a dosage of 150 mg twice daily with fulvestrant or 200 mg twice daily alone, was associated with diarrhea of any grade in 85% to 90% of patients and with grade 3/4 diarrhea in 15% to 20% of patients across clinical studies. Diarrhea typically occurred early (median time to onset, 6 days in MONARCH-2) and was managed with antidiarrheals, followed by dose interruption and dose reduction if needed. More than 70% of the patients in MONARCH-2 who experienced diarrhea did not need dose reduction. Grade 3/4 neutropenia occurred in approximately 25% of patients across studies; this adverse effect can also be managed with dose interruption/dose reduction.
For all three agents, we recommend initiating the approved dose for most patients and then making adjustments for toxicities. Patients should be counseled that dose adjustments may be necessary but that they can still have benefit on a lower dose. Specific guidance about monitoring parameters is provided in Table 2.
Sequencing CDK4/6 Inhibitors With Existing Therapies for HR+/HER2− Advanced Breast Cancer
There are already a number of treatment options for patients with HR+/HER2− advanced breast cancer, including the selective estrogen receptor modulator tamoxifen, nonsteroidal AIs such as anastrozole and letrozole, the selective estrogen receptor degrader fulvestrant, the steroidal AI exemestane (with or without the mammalian target of rapamycin [mTOR] inhibitor everolimus), and chemotherapy. Usually, patients receive sequential endocrine therapies until they develop resistance or experience a visceral crisis that requires chemotherapy.[20] Clinicians must now decide whether to use CDK4/6 inhibitors as part of first- or later-line therapy (approved in both settings) and how to sequence therapies after progression. Clinical evidence to guide these decisions is immature or lacking.
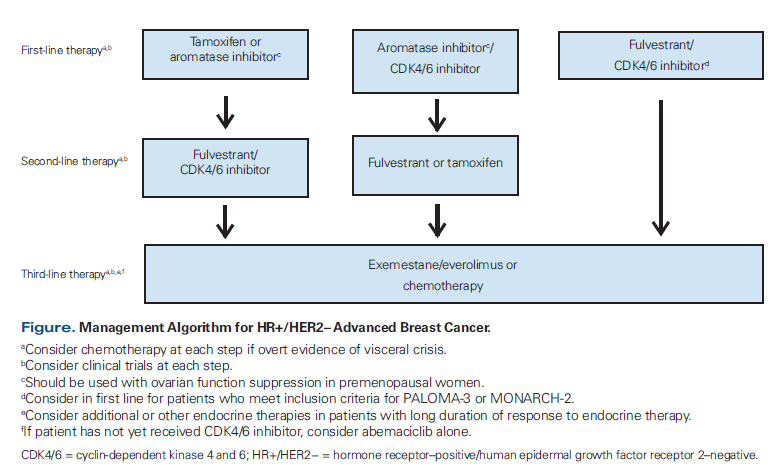
Receiving a CDK4/6 inhibitor during any line of therapy probably confers a survival benefit compared with never receiving one. However, whether receiving a CDK4/6 inhibitor in the first line adds a survival advantage is unknown, and the OS results from PALOMA-2, MONALEESA-2, and MONARCH-3 are eagerly anticipated. Our recommendations for sequencing the CDK4/6 inhibitors with existing therapies are summarized in the Figure.
First-line endocrine therapy
We generally recommend that patients with HR+/HER2− advanced breast cancer receive a CDK4/6 inhibitor along with an AI for first-line treatment, pending OS data. CDK4/6 inhibitors are well tolerated by most women, and even without an OS benefit, a prolonged PFS may delay the onset of more severe disease symptoms and have other benefits. However, select patients with a long disease-free interval, low-volume disease, and/or bone-only disease will likely have a long progression-free interval with endocrine therapy alone. While it is possible that they would do even better with the addition of a CDK4/6 inhibitor, patients should be counseled regarding the treatment schedule for the agents being considered, the need for laboratory and other monitoring, and safety profiles.
The combination of a CDK4/6 inhibitor and an AI is efficacious even for women with visceral disease. Between 45% and 60% of patients enrolled across all first-line studies had visceral disease. In subgroup analyses, these patients derived a benefit similar to that seen in the overall study populations. The combination of a CDK4/6 inhibitor and an AI may be considered even when a rapid tumor response is needed, given its robust ORR (50% to 60% in the first line), but chemotherapy should still be used for a true visceral crisis.[21]
Second-line endocrine therapy
In patients whose disease progresses while receiving a CDK4/6 inhibitor and an AI, we recommend fulvestrant for second-line treatment, although the clinical trials showing fulvestrant’s efficacy in this setting were conducted prior to CDK4/6 inhibitor use.[22,23] Exemestane can also be considered after treatment with a CDK4/6 inhibitor and an AI, or reserved for use in the next line, along with everolimus.[24] There is currently no evidence to support continuing the same CDK4/6 inhibitor or switching to a different CDK4/6 inhibitor after progression.
Patients who received endocrine therapy only (tamoxifen or an AI) as first-line treatment should receive a CDK4/6 inhibitor–based second-line treatment, although for patients with a very long progression-free interval, endocrine therapy alone can again be considered. Those whose disease has progressed while receiving adjuvant or neoadjuvant endocrine therapy or for whom ≤ 12 months has passed since their completion of adjuvant endocrine therapy can receive a CDK4/6 inhibitor with fulvestrant upfront, since they would meet inclusion criteria for PALOMA-3 and/or MONARCH-2.
Third- and later-line endocrine therapy
For patients who received a CDK4/6 inhibitor and fulvestrant or fulvestrant alone for second-line treatment, we would recommend exemestane with the addition of everolimus in the third line, although the efficacy of this combination after receiving a CDK4/6 inhibitor with endocrine therapy is unknown.
KEY POINTS
- The cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors palbociclib, ribociclib, and abemaciclib are approved by the US Food and Drug Administration for first-line, second-line, and later-line treatment of patients with hormone receptor– positive/human epidermal growth factor receptor 2–negative (HR+/HER2−) advanced breast cancer.
- Efficacy data do not support the use of one agent over another; however, their safety profiles differ somewhat and may help select among agents.
- Next steps for CDK4/6 inhibitors include finding predictive biomarkers beyond the estrogen receptor, determining whether to continue these agents beyond disease progression, creating novel combinations with other treatments, and using them for indications other than HR+/HER2− advanced breast cancer.
For patients who have not yet received a CDK4/6 inhibitor but who have received endocrine therapy and chemotherapy, abemaciclib as a single agent may be given, based on data from MONARCH-1. It may also be reasonable to try single-agent abemaciclib before chemotherapy for patients who have exhausted endocrine therapy, although this is different from the approved indication. We do recognize that the number of patients who reach third- or later-line therapy without receiving a CDK4/6 inhibitor will diminish over time and we do not recommend reserving therapy with a CDK4/6 inhibitor for use in this setting.
Next Directions for CDK4/6 Inhibitors
Many questions remain about integrating CDK4/6 inhibitors into clinical practice. These include whether there are biomarkers that may predict response to a CDK4/6 inhibitor, how to determine if a CDK4/6 inhibitor should be continued or switched after progression, whether CDK4/6 inhibitors may be combined with therapies other than endocrine therapies, and whether the use of these agents can be expanded to HR+/HER2− early-stage breast cancer and HR+/HER2+ disease.
Predictive biomarkers for CDK4/6 inhibitors
Approximately 20% of patients will not respond to CDK4/6 inhibitors initially, and all patients will ultimately develop resistance. A better understanding of biomarkers of intrinsic and acquired resistance may help guide therapy. Despite extensive research, estrogen receptor positivity remains the best predictive biomarker for initial response to CDK4/6 inhibitors. PALOMA-1/TRIO-18 initially enrolled two cohorts of patients with HR+/HER2− advanced breast cancer: an unselected group; and a group with either amplification of the cyclin DI gene (CCND1), loss of the p16 gene (INK4A/CDKN2A), or both. The presence of these alterations was not associated with increased benefit from the addition of palbociclib. This was also confirmed in PALOMA-2, where expression levels (whether high or low) of genes in the cyclin D-CDK4/6-Rb pathway did not correlate with benefit from palbociclib plus letrozole.[25] In PALOMA-3, mutations of PIK3CA were detectable in circulating DNA, but not predictive of benefit from palbociclib. Mutations in the ESR1 gene (which encodes estrogen receptor-α) are detectable in 25% to 40% of tumors that become resistant to AI therapy,[26,27] but these were not predictive of benefit or resistance with palbociclib in PALOMA-3.[26] In MONALEESA-2, benefit with ribociclib was maintained irrespective of baseline Rb, Ki-67, or p16 protein expression; or CDKN2A or CCND1 messenger RNA expression levels.[28]
Several ongoing studies with palbociclib (PYTHIA [NCT02536742, NCT03195192]) and ribociclib (NCT03195192) are designed specifically to look at gene- and protein-based predictive biomarkers in an effort to better understand intrinsic and acquired resistance.
Continuing CDK4/6 inhibitors after disease progression
It is not known whether a CDK4/6 inhibitor should be continued beyond the development of disease progression. In two ongoing studies, patients with HR+/HER2− advanced breast cancer whose disease had progressed while receiving a CDK4/6 inhibitor and an AI will receive palbociclib and fulvestrant (NCT02738866) or be randomized to receive ribociclib and fulvestrant or fulvestrant alone (NCT02632045) to determine whether there is a benefit to continuing CDK4/6 inhibitor therapy in this setting.
Novel combinations with CDK4/6 inhibitors
Novel combinations with CDK4/6 inhibitors for patients with HR+/HER2− advanced breast cancer are also being studied. Preclinical data suggest a synergistic effect of inhibiting CDK4/6 and the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway. Several studies are combining a CDK4/6 inhibitor with everolimus and exemestane (NCT02871791, TRINITI-1[NCT02732119], NCT01857193) or with novel inhibitors of the PI3K pathway (NCT02684032, NCT02389842, NCT03128619, NCT03006172, NCT01872260, NCT02088684, NCT02154776). The CDK4/6 inhibitors are also being combined with immunotherapy (programmed death 1 or programmed death ligand 1 inhibitors) in several clinical studies (PACE [NCT03147287], NCT02778685, NCT02779751).
CDK4/6 inhibitors for HR+/HER2− early-stage breast cancer
It is not known whether CDK4/6 inhibitors should be added to adjuvant treatment for patients with HR+/HER2− early-stage breast cancer. Several ongoing studies with palbociclib (PALLAS [NCT02513394]), ribociclib (EarLEE-1 [NCT03078751] and EarLEE-2 [NCT03081234]), and abemaciclib (monarchE [NCT03155997]) are comparing treatment with a CDK4/6 inhibitor plus endocrine therapy vs adjuvant endocrine therapy alone. These studies are enrolling patients with stage II or III disease to determine whether some patients at higher risk for recurrence may benefit from an adjuvant CDK4/6 inhibitor. If the results of these studies are positive, benefit for some patients will need to be balanced against the risks of overtreatment, as is the case with any adjuvant treatment; in addition, novel biomarkers of response could add value.
CDK4/6 inhibitors for HR+/HER2+ advanced breast cancer
Preclinical data suggest that combining a CDK4/6 inhibitor with anti-HER2 therapy may be effective in this setting.[29] Ongoing clinical trials with palbociclib (PATRICIA [NCT02448420], PATINA [NCT02947685]), ribociclib (NCT02657343), and abemaciclib (monarcHER [NCT02675231]) are all examining the benefit of a CDK4/6 inhibitor added to HER2-directed therapy or HER2-directed therapy and endocrine therapy for patients with HR+/HER2+ advanced breast cancer.
Conclusion
The CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib are rapidly changing the treatment paradigm for patients with HR+/HER2− advanced breast cancer. They have demonstrated meaningful improvement in PFS when used for first- or later-line therapy, although OS data are still immature. All three CDK4/6 inhibitors appear to have equivalent efficacy; their somewhat different safety profiles may favor use of one over another in particular patients. We provide a possible framework that clinicians can use to sequence the CDK4/6 inhibitors with existing therapies; future data will better guide this approach. Next steps include identifying biomarkers beyond the estrogen receptor to predict response, determining whether to continue CDK4/6 inhibitors after disease progression, combining these agents with other therapies, and expanding their use into settings other than HR+/HER2− advanced breast cancer.
Financial Disclosure:Dr. Stearns receives research support from AbbVie, Biocept, MedImmune, Novartis, Pfizer, and Puma. Drs. Shah and Nunes have no significant financial interest in or other relationship with the manufacturer of any product mentioned in this article.
References:
1. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417-30.
2. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25-35.
3. Finn RS, Crown J, Lang I, et al. Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2– advanced breast cancer (PALOMA-1; TRIO-18). J Clin Oncol. 2017;35(15 suppl):abstr 1001.
4. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-36.
5. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209-19.
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425-39.
7. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-48.
8. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib + letrozole in hormone receptor-positive (HR+), HER2-negative (HER2–), advanced breast cancer (ABC). J Clin Oncol. 2017;35(15 suppl):1038.
9. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638-46.
10. di Leo A, Toi M, Campone M et al. MONARCH 3: Abemaciclib as initial therapy for patients with HR+/HER2- advanced breast cancer. Presented at the European Society for Medical Oncology 2017 Congress; Madrid; Sep 8–12, 2017. Abstr 236O.
11. Sledge GW, Jr., Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875-84.
12. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res. 2017;23:5218-24.
13. Park IH, Ro J, Lee KS, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol. 2010;28:2705-11.
14. Tripathy D, Sohn J, Im SA, et al. First-line ribociclib vs placebo with goserelin and tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer: results from the randomized phase III MONALEESA-7 trial. Presented at the San Antonio Breast Cancer Symposium; San Antonio, TX; Dec 5–9, 2017. Abstr GS2-05.
15. Loibl S, Turner NC, Ro J, et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22:1028-38.
16. Barroso-Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (Basel). 2016;11:167-73.
17. Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21:1165-75.
18. Hu W, Sung T, Jessen BA, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;22:2000-8.
19. Diéras V, Harbeck N, Joy AA, et al. PALOMA-2: Neutropenia (NP) patterns in patients (Pts) with estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2–) first-line advanced breast cancer (ABC) receiving palbociclib + letrozole (P+L). Presented at the European Society for Medical Oncology 2017 Congress; Madrid; Sep 8–12, 2017. Abstr 291P.
20. Connolly RM, Stearns V. Postmenopausal hormone receptor-positive advanced breast cancer. Oncology (Williston Park). 2013;27:571-2,574,576.
21. Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev. 2003;CD002747.
22. Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-70.
23. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594-600.
24. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-9.
25. Finn RS, Lui Y, Martin M, et al. Comprehensive gene expression biomarker analysis of CDK 4/6 and endocrine pathways from the PALOMA-2 study. San Antonio Breast Cancer Symposium 2017; San Antonio, TX; Dec 5–9, 2017. P2-09-10.
26. Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34:2961-8.
27. Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016;2:1310-5.
28. Andre F, Stemmer SM, Campone M, et al. Ribociclib + letrozole for first-line treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): efficacy by baseline tumor markers. Cancer Res. 2017;77(13 suppl):abstr CT045.
29. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77.