Tumor genomic sequencing has become part of routine oncology practice in many tumor types, in order to identify potentially targetable mutations and to personalize cancer care. Plasma genotyping via circulating tumor DNA analysis is a noninvasive and rapid alternative method of detecting and monitoring genomic alterations throughout the course of disease. Multiple assays have been developed to date, each with different test characteristics and degrees of clinical validation. Here we review the clinical data supporting these different plasma genotyping methodologies, and present a practical approach to the interpretation of the results of these tests. While the clinical application of plasma genotyping has been most extensively validated in the metastatic setting-for the detection of targetable alterations at the time of initial diagnosis or disease progression-this technology holds significant promise across many tumor types and stages of disease. We will also review emerging applications of plasma genotyping that are currently under clinical investigation.
Introduction
Modern targeted therapies have greatly advanced the treatment of many solid tumors. The rational use of these agents requires optimal strategies for the rapid and accurate detection of targetable genomic alterations, both upon initial diagnosis and when acquired resistance to targeted therapies develops. While tissue genotyping has traditionally been considered the standard of care for identifying such genomic alterations, this methodology is limited by often inaccessible or insufficient tumor tissue, and by the risks associated with undergoing serial tumor biopsies. In non–small-cell lung cancer (NSCLC), for example, where molecular testing to guide initial treatment decision making is considered standard of care, clinical studies show that up to 10% to 20% of biopsies are inadequate for molecular testing due to insufficient tissue or amplifiable DNA.[1,2] Furthermore, tumor biopsies are limited by tumor heterogeneity, particularly in the setting of disease resistance, and thus may yield false-negative results.
Plasma genotyping is an emerging technology that can noninvasively and rapidly detect and monitor genomic alterations in cancer patients over time, and which has the potential to overcome some of the limitations associated with tissue genotyping. To date, multiple platforms for plasma genotyping have been tested and validated to various degrees; each has different advantages and limitations, complicating the interpretation and routine integration of these tests into clinical practice.
Biology of Cell-Free DNA
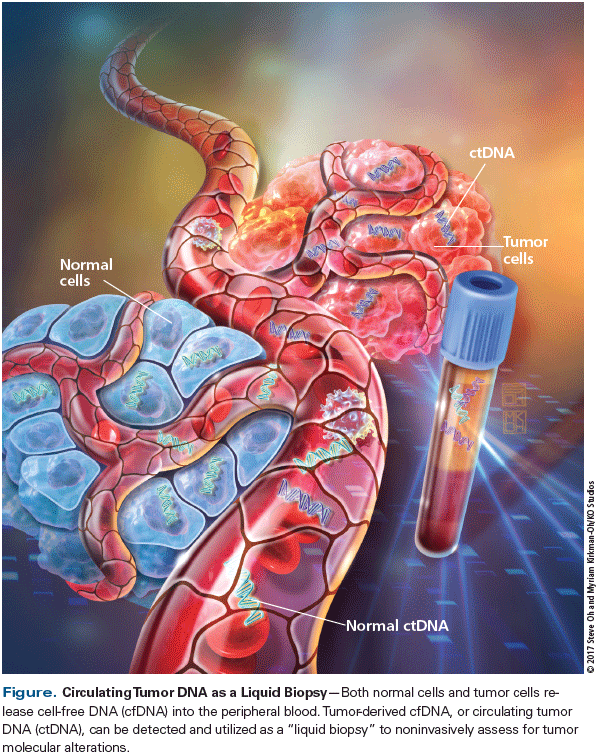
The presence of cell-free DNA (cfDNA) in the plasma was first described in 1948 by Mandel and Metais.[3] It was not until years later that tumor-derived cfDNA-also called circulating tumor DNA (ctDNA)-was discovered, stemming from the finding that cancer patients had higher levels of plasma cfDNA than normal controls.[4,5] The exact mechanism by which DNA is released into the peripheral blood has not been well described; however, it is thought to occur through multiple mechanisms, including tumor cell apoptosis, necrosis, and extracellular vesicle secretion.[6-8] In contrast to genomic DNA, ctDNA is highly fragmented, with most DNA fragments measuring approximately 150 bp in length; this supports the hypothesis that cfDNA originates through cell necrosis or apoptosis.[9,10] Also, peripheral cfDNA exists in low concentrations, with the majority of studies reporting absolute cfDNA levels of less than 100 ng/mL[11]-and only a fraction of this total cfDNA (< 1% of total cfDNA) is actually tumor-derived.[9,12] Thus, highly sensitive methodologies are required to detect ctDNA (Figure).
The concept of ctDNA “shed” is critical to the understanding and interpretation of plasma genotyping assay results, since even the most sensitive assays may not detect a mutation if a tumor is not shedding ctDNA. The degree to which a tumor releases DNA into the peripheral circulation determines the concentration of ctDNA that may be detected in the blood. Factors associated with increased ctDNA shed include mitotic rate, cell death, and tumor vascularization. The extent of metastatic disease burden and sites of metastasis have also been associated with detection of increased ctDNA.[6,7] In particular, the presence of metastasis to the liver or bone has been associated with higher levels of ctDNA in clinical studies.[13]
Current Assays and Validation
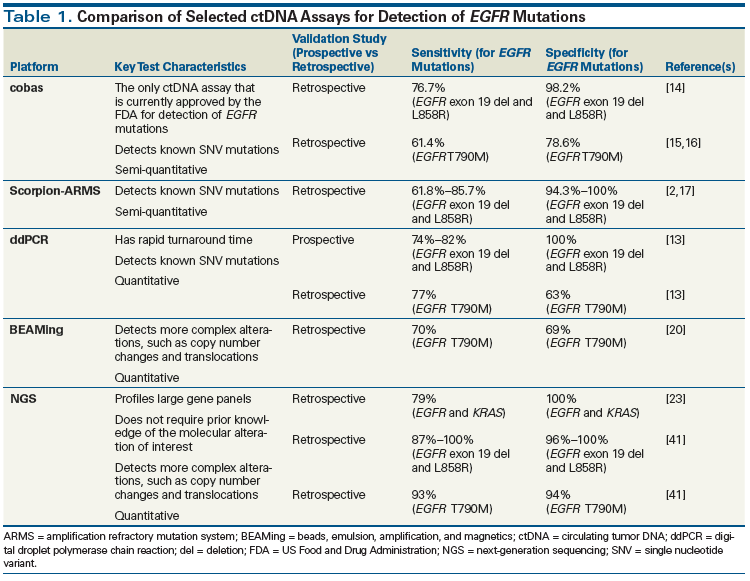
A variety of potential testing platforms for plasma genotyping have been utilized in the past. Each platform has been validated to a different degree and exhibits unique capabilities and test characteristics. Traditional DNA detection methods, such as Sanger sequencing, have generally lacked the sensitivity needed to detect the low levels of ctDNA present in the peripheral blood. Thus, more recent assays have utilized an allele-specific quantitative polymerase chain reaction (PCR)-based platform to enrich for mutant DNA, or have used massive parallel sequencing with a next-generation sequencing (NGS) platform. Most currently validated ctDNA assays have been compared against tissue genotyping. The specificities reported for these tests are consistently high, ranging between 90% and 100%, but their sensitivities are more variable, ranging between 30% and 85%, depending on the testing methodology (Table 1). While few prospective validation studies have been carried out to date, retrospective analysis of samples collected from patients enrolled in prospective clinical trials is a reliable alternative.
Approved assays
The Roche cobas EGFR Mutation Test v2 is the only ctDNA assay that is currently approved by the US Food and Drug Administration (FDA) for the detection of EGFR-sensitizing and resistance mutations in NSCLC. This platform was originally approved by the FDA for the detection of EGFR-sensitizing mutations in tissue. Recently, this approval has been extended to include plasma genotyping for both EGFR-sensitizing mutations and the T790M resistance mutation. The approval of the plasma genotyping assay was based on data from the retrospective analysis of paired plasma and tissue samples from 517 NSCLC patients screened for the ENSURE study, which compared treatment with cisplatin/gemcitabine vs erlotinib in EGFR-mutant NSCLC patients. The cobas assay demonstrated moderate sensitivity and high specificity-76.7% (range, 70.5%–81.9%) and 98.2% (range, 95.4%–99.3%), respectively-for the detection of EGFR-sensitizing mutations.[14] The approval for inclusion of EGFR T790M was based on a retrospective analysis of plasma samples from EGFR-mutant NSCLC patients with acquired resistance to initial kinase inhibitor therapy who were enrolled in the early-phase trials (AURA and AURA2) of the third-generation epidermal growth factor receptor (EGFR) kinase inhibitor osimertinib. When compared with an NGS plasma genotyping assay, the sensitivity and specificity of the cobas assay for detection of EGFR T790M were 91.5% and 91.1%, respectively; however, when compared with tissue genotyping, sensitivity and specificity were lower, at 61.4% and 78.6%, respectively.[15,16] The discordance of these results was hypothesized to be related to the heterogeneity of the presence of resistance mutations in tumor tissue.
PCR-based assays
Many of the earliest attempts at ctDNA genotyping utilized the Scorpion amplification refractory mutation system (Scorpion-ARMS) platform, which uses allele-specific primers with fluorescent probes to amplify target DNA alleles. Kimura et al evaluated the test characteristics of ctDNA analysis using a Scorpion-ARMS platform, comparing it against tissue genotyping in NSCLC patients treated with gefitinib. They demonstrated that cfDNA analysis had the ability to detect EGFR mutations with a sensitivity of 85.7% and a specificity of 94.3%.[17] Similar results have been reported in several other retrospective validation studies that used Scorpion-ARMS technology to assess plasma EGFR mutations in the NSCLC population.[2,18]
Newer PCR methodologies, such as beads, emulsion, amplification, and magnetics (BEAMing) and digital droplet PCR (ddPCR), are characterized by higher sensitivity and specificity, and have the additional capability of quantitating mutant ctDNA levels. A recent prospective clinical study compared a ddPCR-based plasma assay against tissue genotyping for the detection of EGFR and KRAS mutations in patients with advanced NSCLC. This assay demonstrated 100% specificity for the detection of EGFR-sensitizing and KRAS mutations.[13] The median turnaround time for ctDNA testing results was only 3 days (range, 1–7 days), highlighting another advantage of ddPCR technology.[13] BEAMing is a highly specific and sensitive PCR methodology with quantitative capabilities. Bettegowda et al reported ctDNA test characteristics using a BEAMing platform in a cohort of 640 patients across all tumor types and stages, demonstrating variability based on stage and tumor type. Within the cohort of 206 patients with metastatic colorectal cancer, the sensitivity for detection of KRAS mutations was 87.2% and the specificity was 99.2%.[19] ctDNA analysis using BEAMing technology has also been retrospectively validated in studies in NSCLC to detect both EGFR-sensitizing and EGFR resistance mutations.[20,21] One limitation of both ddPCR and BEAMing is their limited ability to assess for more complex alterations, such as translocations or copy number alterations.
NGS assays
NGS technologies are capable of sequencing millions of small DNA fragments in parallel, resulting in highly sensitive and specific detection capabilities. NGS platforms also have the ability to quantify allele frequency and can detect complex alterations such as translocations and copy number changes. Using a multiplex NGS PCR platform to investigate for a panel of 50 cancer-related genes, Couraud et al reported an assay specificity of 86% to 100% and a sensitivity of 58% for the detection of all 50 genes.[22] The ability to detect complex alterations present at low allele frequencies using NGS technology was demonstrated in a study by Paweletz et al. Complex alterations-including rearrangements in ALK, ROS1, and RET; HER2 insertions; and MET amplifications-were detected with 100% specificity and 77% sensitivity.[23] Several commercially available ctDNA assays, including Guardant360 (Guardant Health) and FoundationACT (Foundation Medicine), utilize NGS platforms to investigate for a panel of potentially actionable mutations. Lanman et al validated the Guardant360 platform in a study of 165 patients with advanced solid tumors and reported a sensitivity of 85% and a specificity of > 99%.[24] A subsequent large retrospective analysis of ctDNA testing results from more than 15,000 patients in whom the Guardant360 platform was used reported an accuracy of 87% when plasma genotyping was compared with historical tissue genotyping data from the Cancer Genome Atlas.[25,26]
Guide to Interpretation of Plasma Genotyping Results
With a multitude of commercial assays now available, plasma genotyping is quickly emerging as a routine tool in the armamentarium available for the diagnosis and management of cancer. The clinical utility of plasma genotyping for directing patient care has been most extensively studied at the times of initial diagnosis and disease progression in advanced lung cancer, where molecular testing results directly impact treatment decision making. While tumor tissue genotyping is considered the standard choice for detection of potentially targetable alterations, plasma genotyping may have a role, particularly in settings where either a tissue biopsy is technically infeasible or tissue is inadequate to perform routine genotyping.
Plasma genotyping at initial diagnosis
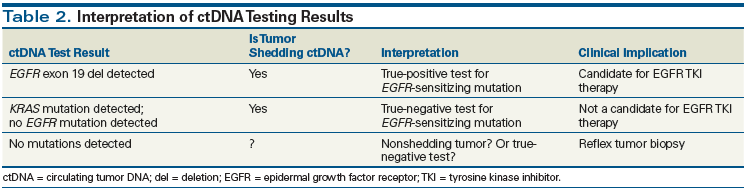
Careful consideration of the biology underlying ctDNA shed and an understanding of the characteristics of the particular assay being used are required to accurately interpret ctDNA test results. As discussed in the aforementioned studies, validated ctDNA assays are characterized by exquisitely high specificity and positive predictive values. As a result, a driver mutation detected by plasma genotyping can be interpreted as a true-positive result. The ability to detect a driver mutation also implies that a tumor is shedding ctDNA at a detectable level. Thus, any negative result in this setting can also be interpreted as a true negative (Table 2). For example, the absence of an EGFR mutation on plasma genotyping, but detection of a KRAS ctDNA mutation, can be interpreted as a true-negative result and a true-positive result, respectively. Interpretation is more complex in the setting where no genomic alterations are detected. In such a case, either the tumor does not possess any of the mutations of interest, or the tumor is not shedding any ctDNA-meaning that the result might be a false negative. Furthermore, while the majority of plasma genotyping assays have near-perfect specificity, sensitivity generally ranges from 70% to 80%, resulting in false-negative tests due to inherent analytic test factors. Thus, a tumor biopsy for confirmatory genotyping should be considered when plasma genotyping results are entirely negative (see Table 2).
Plasma genotyping for resistance mutations
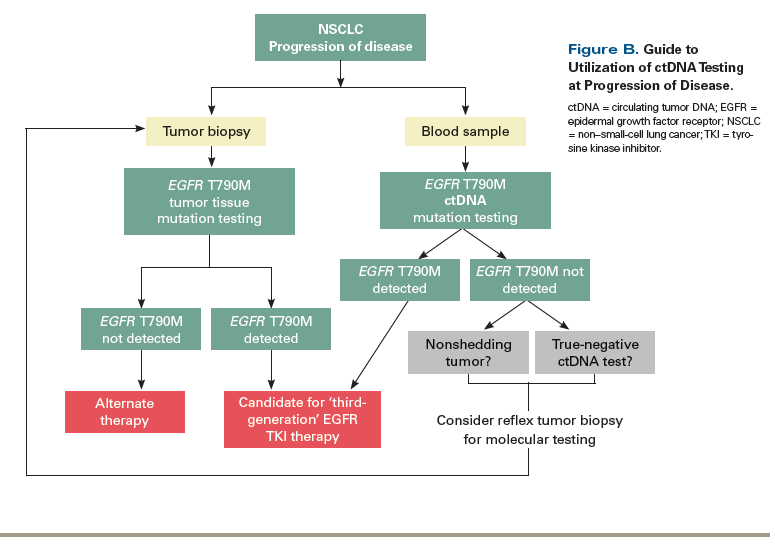
The ability to detect resistance mutations at disease progression is another property of noninvasive testing with plasma genotyping that may be particularly valuable clinically. The detection of EGFR T790M mutations by ctDNA analysis is a key example of this clinical application. Multiple studies in this particular setting have reported lower specificities for the detection of EGFR T790M when plasma genotyping has been compared with tissue genotyping. Thress et al reported sensitivities of 73% and 81% and specificities of 67% and 58% when using the cobas and BEAMing platforms, respectively, for the detection of EGFR T790M. In their analysis they concluded that many of the “false-positive” results were more likely explained by tumor heterogeneity of resistance mutations, rather than being due to a lack of specificity of the assay.[27] Interestingly, subsequent studies have demonstrated that patients treated with osimertinib based on a positive plasma test for the EGFR T790M mutation have outcomes similar to those of patients who are treated based on tissue genotyping results.[20] The recent confirmatory AURA3 study, which randomized patients with T790M-positive NSCLC who had progressed following treatment with at least one EGFR kinase inhibitor to either osimertinib or platinum-based chemotherapy, demonstrated similar outcomes for patients who were T790M-positive by ctDNA testing and those positive by tumor testing, supporting the feasibility of using plasma genotyping in this clinical setting.[28] Thus, with regard to resistance mutation detection, plasma genotyping may be useful to confirm the presence of a targetable resistance mutation such as EGFR T790M. Because the sensitivity of plasma genotyping assays is not perfect, however, a negative ctDNA test should still be followed by a reflex tumor biopsy when feasible, since the latter may identify alternate mechanisms of resistance, such as transformation of disease or complex alterations, which are not as easily identified by plasma genotyping.
Future Applications of Plasma Genotyping
While the utility of ctDNA analysis for the detection of targetable alterations at the time of diagnosis has been well validated, particularly in advanced NSCLC, many other applications of plasma genotyping in cancer are currently being investigated. ctDNA monitoring may have clinical utility throughout the course of disease, from prediagnosis to advanced disease, and in the setting of surveillance as well as that of initial diagnosis. These evolving areas require further prospective study before becoming part of routine clinical practice; however, they hold significant promise for advancing the use of precision medicine in oncology.
Early cancer detection
Early cancer detection using ctDNA testing represents an emerging area of significant clinical interest. The ability to detect low levels of mutant tumor cfDNA up to 2 years prior to the initial diagnosis of cancer has previously been demonstrated in clinical studies.[29] Because mutant ctDNA exists only at low concentrations in patients with early-stage disease, highly sensitive assays are required for testing in this setting. The relationship between tumor stage and the level of detectable ctDNA has been established in several studies, in which ctDNA was reportedly detected in 82% to 100% of patients with stage IV disease, vs in 47% of patients with stage I disease.[19] While advances in ctDNA detection capabilities have made early detection of cancer feasible, one of the main challenges of implementing ctDNA testing as a screening diagnostic is the hazards associated with potential overdiagnosis and false positives. Another limitation and challenge of this approach is that it requires the presence of mutations associated with a high positive predictive value for a cancer diagnosis. It is not currently known how to interpret the detection of mutations in common cancer genes based on low levels of ctDNA, since these may exist even in healthy individuals. For example, mutations in both TP53 and KRAS have been identified in skin biopsies of healthy persons without cancer.[30] Plasma genotyping for cancer detection in the asymptomatic population represents an area of significant promise, but at this time requires further clinical validation before being incorporated into clinical practice.
Longitudinal disease monitoring
Minimal residual disease and recurrence monitoring. Tumor ctDNA has the potential to serve as a noninvasive and specific biomarker for predicting risk of relapse and monitoring minimal residual disease in patients with early-stage cancer who have undergone curative therapy. Additionally, the ability to better risk-stratify patients following curative surgery or chemotherapy may help define subsets of patients who are more likely to benefit from adjuvant therapy, while sparing those who may not require further intensive treatment. This concept was demonstrated in a prospective study of 230 patients with stage II colorectal cancer who underwent ctDNA testing either postoperatively (in those who did not receive adjuvant chemotherapy) or after the completion of adjuvant chemotherapy. Inferior recurrence-free survival was documented in patients in whom ctDNA was detected postoperatively or following the completion of adjuvant chemotherapy.[31] A similar concept was demonstrated in patients with early-stage breast cancer who had completed curative treatment with neoadjuvant chemotherapy followed by surgical resection.[32] The detection of ctDNA in the postoperative setting was highly correlated with relapse risk. Additionally, this study documented a median lead time of 7.9 months prior to the occurrence of clinical relapse in those who underwent serial monitoring.[32]
Clinical Case Study: Utilization of ctDNA Testing to Guide Treatment in Advanced NSCLC
PART A:
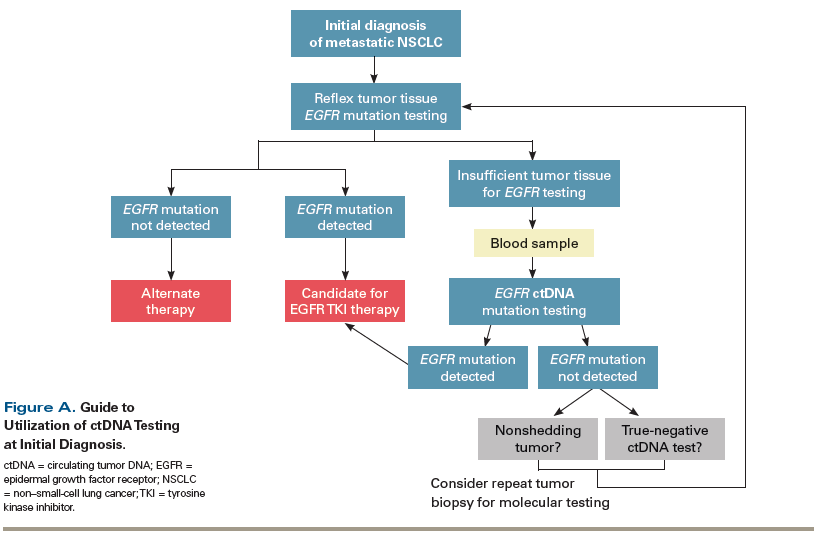
A 60-year-old woman, never smoker, presents with shortness of breath and is diagnosed with metastatic lung adenocarcinoma. Tumor tissue from her initial diagnostic lung biopsy is sent for molecular testing and is returned as “insufficient tumor tissue for analysis.” Blood is sent for circulating tumor DNA (ctDNA) testing using the cobas v2 EGFR assay, and the results are positive for an
EGFR
exon 19 deletion. She is started on first-line therapy with an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor. The detection of a driver mutation with ctDNA testing in this setting can be interpreted as a true-positive result and can be acted upon (Figure A).
PART B:
After 2 years on EGFR kinase inhibitor therapy, the patient’s scans show progression of disease, with multiple new metastatic liver and bone lesions. The cobas v2 EGFR ctDNA assay is used to investigate for an
EGFR
T790M resistance mutation. An
EGFR
T790M mutation is not detected; however, the initial
EGFR
exon 19 deletion is still detected. The detection of the initial
EGFR
mutation suggests that the tumor is shedding ctDNA, and the absence of the T790M mutation can thus be interpreted as a true negative. In a case such as this, a reflex tumor biopsy should be considered to identify alternate resistance mechanisms (Figure B). The patient underwent a tumor biopsy, and molecular testing revealed a
MET
amplification.
One challenge in the development of ctDNA assays in this setting is identifying which specific alterations to investigate for in each patient. Several studies have demonstrated the feasibility of developing personalized ctDNA panels.[33,34] The postoperative setting provides an opportunity to utilize residual tumor tissue to identify patient-specific tumor mutations that may be followed via personalized plasma ctDNA assays. A recent study in 44 patients with early-stage colorectal cancer utilized residual primary tumor tissue to identify tumor mutations, and created patient-specific ctDNA panels that were then followed serially throughout the course of disease. ctDNA was detected before clinical or radiographic relapse in 11 out of 15 patients.[35]
Response to therapy and metastatic disease monitoring. The short half-life of ctDNA and the relative ease of serial sampling make ctDNA an ideal biomarker for monitoring response to therapy in the metastatic setting. Mok et al demonstrated this principle in an exploratory analysis of plasma ctDNA samples obtained from patients with advanced NSCLC who were randomized to receive 6 cycles of gemcitabine and platinum-based chemotherapy plus either sequential erlotinib or placebo.[36] EGFR mutation–specific ctDNA allele levels were quantitated at baseline, after cycle 3, and at progression of disease, and these correlated with response rates and survival outcomes in a subset of patients. Progression-free survival and overall survival were both significantly longer in the patients who had no detectable mutant EGFR alleles compared with those who did have detectable EGFR-mutant alleles after 3 cycles of treatment. This study provides evidence that changes in levels of EGFR-mutant alleles may predict benefit of continued treatment with EGFR-targeted therapy in patients with advanced NSCLC, although further prospective study in larger trials is required.[36]
KEY POINTS
- The majority of circulating tumor DNA (ctDNA) assays have high specificity but more moderate sensitivity. Thus, the detection of a driver mutation by a validated ctDNA assay can generally be interpreted as a true-positive result and can be acted upon.
- A negative ctDNA test result is more difficult to interpret, and can represent either a true-negative result or a tumor that is simply not shedding any ctDNA. A tumor biopsy should be considered in such cases.
- ctDNA testing holds significant potential as a tool that can be applied throughout the course of disease, from cancer detection to monitoring of response to therapy, although routine use in these settings still requires further investigation.
Although plasma genotyping has been most extensively studied in advanced NSCLC, where molecular testing results have a direct impact on the initial treatment decision, clinical studies in other tumor types have similarly explored the role of ctDNA as a biomarker of disease activity and treatment response. In a proof-of-concept analysis, Dawson et al correlated dynamic changes in ctDNA levels with radiographic responses to therapy in patients with advanced breast cancer.[37] ctDNA was the earliest marker of disease activity in 10 of the 19 patients evaluated, with increases in ctDNA levels observed, on average, 5 months earlier than documented radiographic disease progression. Additionally, ctDNA levels showed a closer correlation with radiographic changes in tumor burden than cancer antigen 15-3 levels or circulating tumor cells in this study.[37] Similarly, in colorectal cancer, Tie et al found that early changes in ctDNA levels during chemotherapy treatment were predictive of radiographic responses.[38] In their prospective study of 53 patients with metastatic colorectal cancer receiving first-line chemotherapy, a reduction in ctDNA levels before cycle 2 (median, 5.7-fold reduction) correlated with radiographic response at 8 to 10 weeks.[38] While the results of these studies are promising and suggest that ctDNA may be a more sensitive biomarker of disease response than current standard imaging techniques, the use of ctDNA in this setting requires additional prospective validation. Also, further characterization of the threshold of change in ctDNA needed to predict response in each tumor type must be better defined before ctDNA testing can be used for clinical decision making in this setting.
A tool for drug development
Early-phase drug development is another scenario where a noninvasive and rapid method of assessing disease response and mechanisms of resistance could have significant clinical impact. Currently, early-phase clinical trials are time- and resource-intensive, often requiring serial tumor biopsies and frequent imaging in order to obtain the maximal clinical and biological information from each treated patient. Given the sensitivity of current ctDNA platforms, changes in ctDNA levels may be used to assess response to treatment as early as several weeks after initiation of therapy, considerably earlier than when radiographic responses can be used for such assessment. Another potential application of plasma genotyping that is particularly relevant to targeted therapy trials is the ability of ctDNA assays to noninvasively monitor for and detect resistance mechanisms in real time. Frenel et al demonstrated the ability of serial ctDNA monitoring to assess disease response and clonal evolution in response to targeted therapies in an early-phase clinical trial setting.[39] Another prime example of the usefulness of plasma genotyping for the detection of novel resistance mechanisms in an early-phase clinical trial setting is the discovery of the EGFR C797S mutation, which mediates resistance to third-generation EGFR kinase inhibitors. In an exploratory analysis, plasma genotyping from 7 EGFR-mutant patients who were enrolled in the phase I AURA study and who developed acquired resistance to osimertinib therapy revealed an EGFR C797S resistance mutation in 1 patient. Subsequent analysis of serial plasma samples from 15 patients treated with osimertinib, using a ddPCR assay, detected the presence of an acquired C797S resistance mutation in 6 of these patients, highlighting the importance of this resistance mechanism.[40]
Genotyping of other bodily fluids
One of the main advantages of plasma genotyping is its noninvasive nature, which facilitates serial testing over time. ctDNA is also released into other body fluids and thus may be assessed and monitored in these other fluid types. The feasibility of urine genotyping was demonstrated by Reckamp et al, who used a mutation enrichment NGS platform to compare rates of detection of EGFR mutations in both urine and plasma against tissue genotyping in EGFR-mutant NSCLC patients receiving rociletinib in the TIGER-X trial.[41] Using tumor tissue as a reference standard, the sensitivity of urine genotyping across all urine collections was 72% for detection of T790M mutations, 75% for L858R mutations, and 67% for exon 19 deletions. The sensitivity of urine testing was increased when a higher volume (at least 90–100 mL) of urine was analyzed, with results comparable to those reported with plasma genotyping. As with plasma genotyping, urine ctDNA analysis was highly specific, with a specificity of 96% for detection of T790M mutations, 100% for L858R mutations, and 94% for exon 19 deletions.[41] Thus, urine genotyping may be another feasible means of detecting and analyzing ctDNA. Recent studies have also explored the possibility of saliva genotyping, which would require a saliva sample of only 20–40 μL to noninvasively detect targetable mutations.[42,43]
Given the sensitivity of ctDNA assays, there may also be a role for this technology in disease detection and monitoring in bodily fluids where standard testing using cytology has poor sensitivity. For example, ctDNA testing of cerebrospinal fluid (CSF) may be a more sensitive means of detecting and monitoring metastatic disease in the central nervous system. The feasibility of such an approach was demonstrated using a digital PCR-based assay to quantify BRAF V600E– or V600K–mutant ctDNA in the CSF of patients with metastatic melanoma or systemic histiocytosis. Mutant ctDNA was detected in 6 of 11 patients analyzed, whereas CSF cytology was positive in only 2 of these 11 patients.[44] Studies in the NSCLC population have similarly shown the feasibility of detecting EGFR mutations in the CSF of patients with known brain metastases.[45,46] Given the difficulty of obtaining tissue from central nervous system tumors, liquid biopsy with ctDNA also offers a promising alternative for identifying mechanisms of disease resistance.[47]
Conclusion
Plasma genotyping using ctDNA is a rapidly evolving and promising technology with potential for significant clinical impact in the field of precision oncology. In general, validated plasma genotyping assays may be used for clinical decision making when positive; however, a completely negative test should still be followed up with reflex tissue genotyping. Because of its noninvasive nature, ctDNA testing has many potentially promising applications throughout the course of disease, including early disease detection, risk stratification following curative treatment, and disease response monitoring over time. These applications require ongoing prospective study before being incorporated into routine care.
Financial Disclosure:Dr. Sacher has received travel funding from AstraZeneca and Genentech-Roche, and has served as principal investigator on clinical trials for both firms. Dr. Komatsubara has no financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169-80.
2. Douillard JY, Ostoros G, Cobo M, et al. Gefitinib treatment in EGFR mutated Caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol. 2014;9:1345-53.
3. Mandel P, Metais P. Les acides nucleiques du plasma sanguin chez l’ homme [The nucleic acids in blood plasma in humans]. C R Seances Soc Biol Fil. 1948;142:241-3.
4. Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646-50.
5. Stroun M, Anker P, Maurice P, et al. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46:318-22.
6. Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659-65.
7. Stroun M, Lyautey J, Lederrey C, et al. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta. 2001;313:139-42.
8. Anker P, Stroun M, Maurice PA. Spontaneous extracellular synthesis of DNA released by human blood lymphocytes. Cancer Res. 1976;36:2832-9.
9. Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci USA. 2005;102:16368-73.
10. Mouliere F, Rosenfeld N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc Natl Acad Sci USA. 2015;112:3178-9.
11. Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer-a survey. Biochim Biophys Acta. 2007;1775:181-232.
12. Yong E. Cancer biomarkers: written in blood. Nature. 2014;511:524-6.
13. Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016;2:1014-22.
14. US Food and Drug Administration. Cobas EGFR mutation test v2. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm504540.htm. Accessed July 17, 2017.
15. Jenkins S, Yang J, Ramalingam S, et al. 134O_PR: Plasma ctDNA analysis for detection of EGFR T790M mutation in patients (pts) with EGFR mutation-positive advanced non-small cell lung cancer (aNSCLC). J Thorac Oncol. 2016;11:S153-S154.
16. Jenkins S, Yang JC, Ramalingam SS, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:1061-70.
17. Kimura H, Suminoe M, Kasahara K, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA). Br J Cancer. 2007;97:778-84.
18. Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 2012;7:115-21.
19. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24.
20. Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:3375-82.
21. Karlovich C, Goldman JW, Sun JM, et al. Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase I study of rociletinib (CO-1686). Clin Cancer Res. 2016;22:2386-95.
22. Couraud S, Vaca-Paniagua F, Villar S, et al. Noninvasive diagnosis of actionable mutations by deep sequencing of circulating free DNA in lung cancer from never-smokers: a proof-of-concept study from BioCAST/IFCT-1002. Clin Cancer Res. 2014;20:4613-24.
23. Paweletz CP, Sacher AG, Raymond CK, et al. Bias-corrected targeted next-generation sequencing for rapid, multiplexed detection of actionable alterations in cell-free DNA from advanced lung cancer patients. Clin Cancer Res. 2016;22:915-22.
24. Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One. 2015;10:e0140712.
25. Zill OA, Banks KC, Jackson C, et al. Comparison of over 10,000 clinical NGS circulating tumor DNA profiles to tissue-derived genomic compendia. Presented at the Annual Meeting of the American Association for Cancer Research; April 16-20, 2016; New Orleans, LA. Abstr 4343.
26. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl):abstr LBA11501.
27. Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90:509-15.
28. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629-40.
29. Gormally E, Vineis P, Matullo G, et al. TP53 and KRAS2 mutations in plasma DNA of healthy subjects and subsequent cancer occurrence: a prospective study. Cancer Res. 2006;66:6871-6.
30. Martincorena I, Roshan A, Gerstung M, et al. Tumor evolution: high burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880-6.
31. Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra92.
32. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133.
33. Leary RJ, Kinde I, Diehl F, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14.
34. McBride DJ, Orpana AK, Sotiriou C, et al. Use of cancer-specific genomic rearrangements to quantify disease burden in plasma from patients with solid tumors. Genes Chromosomes Cancer. 2010;49:1062-9.
35. Ng SB, Chua C, Ng M, et al. Individualised multiplexed circulating tumour DNA assays for monitoring of tumour presence in patients after colorectal cancer surgery. Sci Rep. 2017;7:40737.
36. Mok T, Wu YL, Lee JS, et al. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin Cancer Res. 2015;21:3196-203.
37. Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199-209.
38. Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26:1715-22.
39. Frenel JS, Carreira S, Goodall J, et al. Serial next-generation sequencing of circulating cell-free DNA evaluating tumor clone response to molecularly targeted drug administration. Clin Cancer Res. 2015;21:4586-96.
40. Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21:560-2.
41. Reckamp KL, Melnikova VO, Karlovich C, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. 2016;11:1690-700.
42. Wei F, Lin CC, Joon A, et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 2014;190:1117-26.
43. Pu D, Liang H, Wei F, et al. Evaluation of a novel saliva-based epidermal growth factor receptor mutation detection for lung cancer: a pilot study. Thorac Cancer. 2016;7:428-36.
44. Momtaz P, Pentsova E, Abdel-Wahab O, et al. Quantification of tumor-derived cell free DNA (cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget. 2016;7:85430-6.
45. Zhao J, Ye X, Xu Y, et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol. 2016;78:1305-10.
46. Yang H, Cai L, Zhang Y, et al. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J Mol Diagn. 2014;16:558-63.
47. Pentsova EI, Shah RH, Tang J, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34:2404-15.