CLEOPATRA: Pertuzumab Ups Survival by 16 Months in HER2-Positive Breast Cancer
Data from the CLEOPATRA trial show that pertuzumab adds nearly 16 months to median survival times when used with trastuzumab and chemo in HER2-positive breast cancer, according to final results presented at the ESMO 2014 Congress.
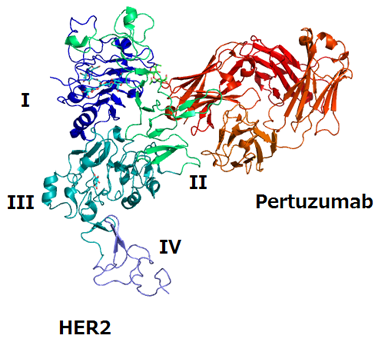
Structure of HER2 extracellular domain and pertuzumab complex; source: Takuma-sa, Wikimedia Commons
Final results from the pivotal CLEOPATRA trial show that pertuzumab adds almost 16 months to median survival times when combined with trastuzumab and chemotherapy compared with trastuzumab and chemotherapy alone in HER2-positive metastatic breast cancer.
“The survival improvement of nearly 16 months observed in CLEOPATRA is unprecedented among studies of metastatic breast cancer,” said Sandra Swain, MD, of the Washington Cancer Institute in Washington, DC, in a press release. The new analysis, with follow-up out to a median of 50 months, was presented at the European Society for Medical Oncology (ESMO) 2014 Congress in Madrid.
Pertuzumab, a monoclonal antibody, was approved by the Food and Drug Administration in 2012 for HER2-positive advanced/metastatic breast cancer in combination with trastuzumab and docetaxel. A year later the agency granted priority review for the drug as a neoadjuvant therapy in these patients, and granted approval for that indication in September 2013.
The study included 808 patients with HER2-positive metastatic breast cancer; patients received trastuzumab and docetaxel along with either pertuzumab or placebo. Earlier interim reports of improved survival with pertuzumab were maintained, with a hazard ratio in favor of the study drug of 0.68 (95% CI, 0.56-0.84; P = .0002). The median overall survival for placebo patients was 40.8 months compared with 56.5 months for pertuzumab patients, for a difference of 15.7 months.
Following a previous report of improved outcomes with pertuzumab, the trial allowed crossover from the placebo arm; 48 patients did cross over. The safety profile of the pertuzumab combination was consistent with previous reports both in the overall cohort and in the crossover patients. The survival benefit was consistent across various patient subgroups, and previously reported progression-free survival benefit was also maintained at this analysis.
“The median overall survival data… is remarkable,” said Javier Cortes, MD, of the Vall D’Hebron Institute of Oncology in Barcelona, Spain, another author on the study. He noted that the improvement in median overall survival exceeded the improvement in progression-free survival, possibly related to the mechanisms of action of monoclonal antibodies like pertuzumab.
“We should consider this combination as the standard of care for our patients,” Cortes said. “I can see no reason to justify the use of trastuzumab without pertuzumab.” Further improvements will need a better understanding of the mechanism of action for this drug combination.