Mantle cell lymphoma is an aggressive B-cell non-Hodgkin lymphoma that is often considered incurable. Different clinical and biological biomarkers can be utilized to categorize this lymphoma into various risk levels. Several randomized trials reported in 2015 shed light on the optimal induction therapy. Recent advances include: (1) identification of new pathways to target, (2) novel therapeutics to treat patients with relapsed/refractory disease, and (3) monitoring of minimal residual disease and adoption of a maintenance therapy approach to prevent relapses post induction or post stem cell transplantation. Due to the efforts of translational/clinical research, the overall survival of patients with mantle cell lymphoma has increased and should continue to improve.
Introduction
Mantle cell lymphoma (MCL), an aggressive B-cell non-Hodgkin lymphoma, is considered incurable. It represents about 4% of lymphomas in the United States.[1] The median age at time of disease presentation is about 60 years, and there is a disposition towards male predominance. The majority of patients present with advanced-stage disease involving the bone marrow and peripheral blood, as well as diffuse lymphadenopathy. Some patients also present with splenomegaly and extranodal disease such as lymphomatous polyposis of the large bowel.[2] Lymphoma- associated inflammatory or B symptoms such as fever, chills, and night sweats are common. Diagnosis is based upon biopsy of a lymph node or bone marrow showing sheets of monomorphic, small to medium-sized lymphoid cells.[3] There are four cytologic variants, including small cell, mantle zone, diffuse, and blastoid variant.[4] The pathognomonic feature of MCL is overexpression of cyclin D1 due to chromosomal translocation t(11;14)(q13;q32), a phenomenon observed in over 95% of cases.[4] This determination can be made by immunohistochemistry or fluorescence in situ hybridization analysis. In cases where cyclin D1 is not overexpressed, cyclin D2 and D3 overexpression can be found.[5] The initial patient workup should include complete blood count (CBC); comprehensive chemistry profile; lactate dehydrogenase (LDH) levels; bone marrow biopsy; and a CT scan of the neck, chest, abdomen, and pelvis-or alternatively, 18-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT. Endoscopic evaluation of the gastrointestinal tract should be considered, especially if the patient presents with clinical symptoms or anemia.
Clinical Presentation
A 54-year-old man presented to a primary care office with a lump on the right side of his neck. He reported that the lump had existed for 2 months, and said he had experienced fever, chills, and night sweats for the past month. Upon examination of the patient, the primary care physician found swollen right cervical lymph nodes, bilateral axillary lymphadenopathy, and enlarged right inguinal lymph nodes. Furthermore, the CBC showed an increased white blood cell (WBC) count, with lymphocytosis and several unclassified cells. Pathology results from a core needle biopsy of the patient’s right axillary lymph node showed the blastoid variant type of MCL. The patient was then referred to a hematologist, who performed an FDG-PET scan. The scan showed increased standardized uptake values, as well as discrete masses in the right cervical neck region, bilateral axillary lymphadenopathy, retroperitoneal lymphadenopathy, increased uptake in the spleen, and right inguinal lymphadenopathy. A bone marrow biopsy revealed MCL involvement. The peripheral blood was submitted to flow cytometry, and immunophenotyping showed a phenotype that was CD19- , CD20- , and CD51-positive and CD10- , CD23- , and BCL6-negative. This patient was diagnosed with stage IVB MCL.
Clinical Management
Unlike other types of lymphomas, treatment of MCL is not usually defined by Ann Arbor staging, since the majority of patients present with advanced-stage disease. Typically, the treatment of newly diagnosed MCL can be tailored toward younger patients (or those < 65 years old who can tolerate intensive chemotherapy) and elderly patients (or those > 65 years old who are ineligible for high-dose therapy). Clinical and biologic prognostic factors should also be taken into consideration when deciding on the timing of therapy and which treatment regimen to use.
Prognostic factors
Mantle Cell International Prognostic Index (MIPI). The MIPI was formulated by the European Mantle Cell Lymphoma Network; it uses age, Eastern Cooperative Oncology Group performance status, LDH levels, and WBC count to stratify patients into three different risk groups.[6] Low-risk patients tend to do well, with a median overall survival (OS) time after diagnosis not reached and a 5-year OS rate of 60%. Intermediate-risk patients have a median OS of 51 months, and high-risk patients have a median OS of 29 months. This index has been validated by other groups, and variations have been used in retrospective and prospective trials.[7] Besides the MIPI, the biomarkers SOX11, Ki-67, p53, and p16 have also been shown to have prognostic value. SOX11 is a transcription factor, and its absence has been associated with an indolent form of MCL.[8] High Ki-67 proliferation and deletion of p53 and p16 have been shown to be associated with blastoid variant MCL, which has a worse OS outcome.[9] Gene expression profiling (GEP) has identified preliminary molecular predictors. A recent study identified RAN, MYC, TNFRSF10B, POLE2, and SLC29A2 as predictors of survival in a small series of patients with MCL.[10] Patients with increased expression of all five genes had inferior survival. Since GEP is not practical to perform on a large scale, this method needs to be explored further before it can be applied in clinical management. Although the genetic hallmark of MCL is cyclin D1 overexpression due to t(11;14) translocation, differential overexpression of cyclin D1 can be seen in various patient samples. Wiestner et al identified patients with a truncated cyclin D1 3' untranslated region as having inferior outcomes.[11] This can be due to altered miR-16-1 regulation, as shown by Chen et al.[12]
Upfront treatment
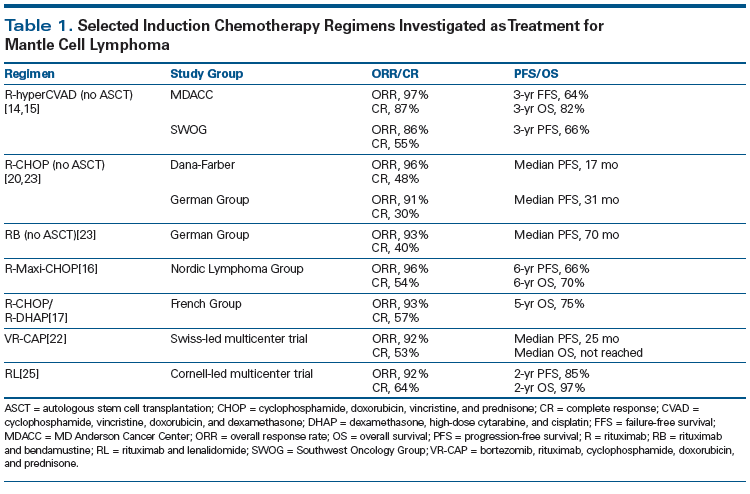
For younger, fit patients with MCL, the therapeutic strategy has been to use aggressive induction chemotherapy followed by autologous stem cell transplantation (ASCT). The benefit of consolidative ASCT has been shown by the European Mantle Cell Lymphoma Network. Their trial compared consolidative ASCT against maintenance treatment with interferon, and showed improved progression-free survival (PFS) in the ASCT arm (median PFS, 39 vs 17 months; P = .0108).[13] Although the role of ASCT in younger patients with MCL is clear, the optimal induction chemotherapy has not been defined. Romaguera et al reported outcomes of the rituximab (R)-hyperCVAD regimen at the University of Texas MD Anderson Cancer Center.[14] This multi-agent regimen contains hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose cytarabine and methotrexate for 6 to 8 cycles. The complete response (CR) rate was 87% and the overall response rate (ORR) was 97%, with a 3-year failure-free survival rate of 64% and OS rate of 82%.[14] However, the results were not quite as favorable when tested in a multicenter setting, with a reported ORR of 86%, a CR rate of 55%, and an estimated 3-year PFS rate of 66%.[15] In addition, 90% of the patients had grade 4 hematologic toxicities, and only 61% of the patients could finish the full course of treatment.[15] Other alternatives to R-hyperCVAD include the Nordic regimen (R-Maxi-CHOP [cyclophosphamide, doxorubicin, vincristine, and prednisone] alternating with high-dose cytarabine without methotrexate). Geisler et al showed that this induction followed by ASCT yielded a 6-year PFS rate of 66% and OS rate of 70%.[16] Delarue et al developed a regimen comprising 3 cycles of R-CHOP followed by 3 cycles of R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) followed by ASCT.[17] The reported results are quite promising, with a CR rate of 57%, ORR of 93%, and 5-year OS rate of 75%. A recent Southwest Oncology Group (SWOG)-led US Intergroup trial compared R-bendamustine (RB) followed by ASCT vs R-hyperCVAD followed by ASCT in younger patients with untreated MCL. The results were reported in June 2015 at the 13th International Conference on Malignant Lymphoma in Lugano, Switzerland[18] and updated at the 2015 American Society of Hematology (ASH) Annual Meeting.[19] It was shown that, although treatment with RB vs R-hyperCVAD was associated with similar ORR, CR, 2-year PFS, and OS rates, patients who received R-hyperCVAD had significantly more instances of myelosuppression, including anemia (59% vs 9% with RB), neutropenia (65% vs 34%, respectively), and thrombocytopenia (71% vs 17%, respectively). R-hyperCVAD also resulted in more stem cell collection failures (29% vs 6%), and its use led to premature closure of the study. At this time, there are no clear data to show which induction regimen should be the standard of care in younger patients with MCL. However, the most appropriate strategy may be to choose the regimen associated with a high CR rate while sparing patients from treatment toxicity so that they can reach eligibility for ASCT.
Regarding older patients who cannot tolerate intensive chemotherapy or ASCT, there are a number of options. Historically, R-CHOP chemotherapy was used, with an ORR of 96%, a CR rate of 48%,[20] and a PFS duration of about 17 months. R-CHOP has also been shown to be superior to R-FC (fludarabine and cyclophosphamide) by a group in Germany. In that study, the 4-year OS rate was 65% for R-CHOP vs 50% for R-FC.[21] A recent study comparing R-CHOP vs VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) showed a 59% improvement in PFS, in favor of VR-CAP, and the median OS rate was not reached vs 56.3% with R-CHOP.[22] Another option besides R-CHOP–based therapy is the use of bendamustine. Rummel et al studied the combination of RB in patients with untreated MCL, reporting an ORR of 75% and a CR rate of 50%, with minor hematologic toxicities.[23] This regimen was then compared against R-CHOP in a large randomized noninferiority trial in Europe, which showed ORRs similar to those achieved with R-CHOP (93% vs 91%), as well as comparable CR rates (40% vs 30%); however, patients treated with RB had improved median PFS (70 vs 31 months), fewer hematologic toxicities (30% vs 68%), and fewer infections (37% vs 50%).[23] Because of recent studies, many physicians are opting to use RB or VR-CAP rather than R-CHOP. Since elderly patients are not eligible to undergo ASCT, the role of maintenance therapy has been evaluated post induction. The study from Germany that compared R-CHOP vs R-FC also evaluated the role of maintenance rituximab vs interferon. The researchers found that the study arm with the best PFS and OS was R-CHOP followed by maintenance rituximab, with a 4-year OS rate of 87%.[21] The role of maintenance rituximab has been confirmed further by meta-analysis of three large randomized trials showing an improvement in PFS with rituximab (hazard ratio [HR], 0.60 [95% CI, 0.44–0.82]).[24] Although no studies have evaluated the role of maintenance rituximab post RB or VR-CAP, most physicians would administer maintenance therapy to patients regardless of the type of induction regimen used.
Besides combination chemotherapy induction regimens, another option exists for elderly patients who may choose not to undergo multi-agent chemotherapy. Ruan et al studied lenalidomide plus rituximab as initial treatment for MCL. This single-arm multicenter trial showed an ORR of 92%, a CR rate of 64%, and a 2-year PFS rate of 85%.[25] Patients were treated with this regimen for at least 36 cycles or until disease progression. Although this was not a randomized trial comparing lenalidomide plus rituximab vs multi-agent chemotherapy, the ORR and PFS are certainly on par with historical controls. Table 1 shows a list of induction regimens discussed.
A small number of patients with MCL have indolent disease. Martin et al evaluated the watch-and-wait approach (similar to that followed in the management of patients with follicular lymphoma) for patients with asymptomatic disease or those who have low MIPI, or for elderly patients with MCL. The results show that the median time to treatment was about 12 months (range, 4–128 months),[26] and the survival profile of the observation group appeared improved compared with that of the early treatment group. The majority of patients received an R-CHOP–like treatment at the time induction therapy was given. This outcome suggests that the watch-and-wait approach may be appropriate for a subset of patients with very indolent disease.
The patient in our initial case presentation received 6 cycles of RB followed by ASCT. He also enrolled on our trial using rituximab plus bortezomib as maintenance therapy post ASCT. He is currently doing well and still in remission.
Relapsed disease
Before the recent advances in novel therapeutics, patients with relapsed MCL were treated with multi-agent salvage chemotherapy, much like the treatment of diffuse large B-cell lymphoma (DLBCL), with regimens such as RICE (rituximab, ifosfamide, carboplatin, and etoposide), R-ESHAP (rituximab, etoposide, methylprednisolone, high-dose cytarabine, and cisplatin), R-DHAP, or gemcitabine-based strategies. RB has been used in the setting of relapsed disease and has been combined with cytarabine (R-BAC). The ORR of these agents appears to be high, at 80% to 90%, with a CR rate of 60% to 70%.[27,28] However, adverse events are common, including cytopenias.
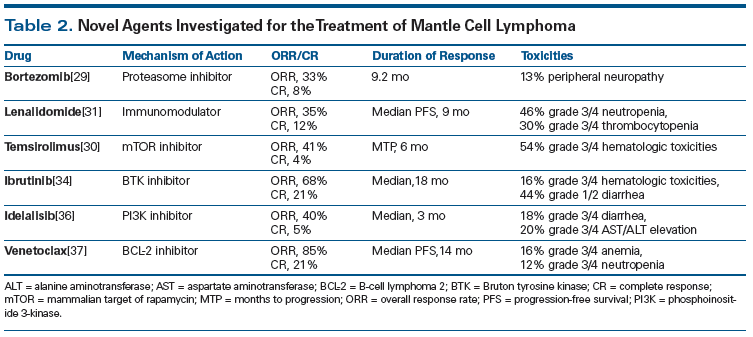
A variety of novel therapies have been tested in recent years and have shown efficacy in relapsed MCL. Bortezomib, a proteasome inhibitor, has demonstrated an ORR of 33% and a CR rate of 8% in this setting, with the most common grade 3 or higher toxicity being peripheral sensory neuropathy in 13% of the patients.[29] Temsirolimus, a mammalian target of rapamycin (mTOR) inhibitor, yielded an ORR of 41% and a CR rate of 4% in a phase II study of patients with relapsed/refractory MCL.[30] Patients treated with the immunomodulatory agent lenalidomide were shown in a phase II trial to have an ORR of 35%, a CR rate of 12%, and median PFS of 9 months.[31] Wang et al combined lenalidomide with rituximab in the setting of relapsed MCL and found that the regimen yielded an ORR of 58% and a CR rate of 33%.[32] Perhaps the most exciting agent to gain approval from the US Food and Drug Administration in this setting is the Bruton tyrosine kinase inhibitor ibrutinib.[33] It was shown to achieve a 68% ORR and a 21% CR rate in patients with relapsed/refractory MCL. These study results are similar to outcomes reported for patients with MCL who have received prior bortezomib vs those who have not, indicating that ibrutinib achieves a response in bortezomib-resistant patients. The median duration of response was 17.5 months, the 2-year PFS rate was 31%, and the 2-year OS rate was 47%. Overall, ibrutinib was well-tolerated; 16% of patients had grade 3/4 hematologic toxicities and 44% had grade 1/2 diarrhea.[34] Although the approval of ibrutinib has benefited many patients with MCL, it has become clear with long-term follow-up that these patients can still experience disease progression while on treatment. The median PFS is 13 months.[35]
Phosphoinositide 3 (PI3)-kinase inhibitors represent another class of drugs that have been investigated in relapsed/refractory MCL. In a phase I study, idelalisib, which like ibrutinib targets the B-cell receptor pathway, generated a 40% ORR, a CR rate of 5%, and a 3-month median duration of response.[36] At the 2015 ASH Annual Meeting, results were presented from a phase I study of venetoclax, a drug that has generated some excitement in this setting. Gerecitano et al evaluated venetoclax, a B-cell lymphoma 2 inhibitor, in patients with relapsed/refractory MCL, follicular lymphoma, chronic lymphocytic leukemia, and DLBCL.[37] Patients with relapsed/refractory MCL appeared to have the most favorable ORR at 75%, with a CR rate of 21%. We anxiously await the completion of that trial and the phase II trial in patients with MCL. At City of Hope, we will evaluate the combination of ibrutinib and venetoclax in patients with relapsed/refractory disease. Table 2 shows a list of novel therapeutics used in relapsed/refractory MCL.
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"47768","attributes":{"alt":"","class":"media-image","id":"media_crop_1839882975415","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"5657","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","style":"height: 144px; width: 144px;","title":" ","typeof":"foaf:Image"}}]]
John P. Leonard, MD,
Meyer Cancer Center, Weill Cornell Medical Center and New York-Presbyterian Hospital, New York, New YorkWhat Are Key Challenges in the Management of Mantle Cell Lymphoma?Various advances have been made in mantle cell lymphoma (MCL). Unfortunately, however, only a few of these have been definitively associated with overall survival benefits. Much of the clinical care of patients with MCL remains based on patient and physician preferences and medical judgment regarding quality of life, rather than definitive improvements in outcome shown by comparative studies. Chen and colleagues report on treatment advances in MCL that herald progress but are limited in their ability to specifically guide therapy for individual patients in an evidence-based fashion. The adoption of the Mantle Cell Lymphoma International Prognostic Index provides a basis for predicting outcomes but does not guide the practitioner on treatment choice, in part because studies of intensive treatment have been enriched for lower-risk patients. Novel agents are useful in the relapsed setting, but with the exception of rituximab maintenance and bortezomib therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), it remains unclear how or if the newer drugs should be added to upfront therapy in older patients.How Can We Improve MCL Research and Treatment?Two critical issues are key in the planning of clinical trials in MCL. The appropriate use of minimal residual disease (MRD) monitoring to guide the use of intensive therapy (eg, whether or not patients who are MRD-positive or -negative after induction should receive stem cell transplant consolidation) or maintenance therapy will be key in future individualized treatment strategies. Additionally, better metrics of quality of life are needed to assess the value of intensive vs chronic or maintenance therapies in the absence of survival improvement. Patients with MCL clearly are doing better, but they deserve better clinical data to guide them through an expanding array of novel treatment options.
The Role of Stem Cell Transplantation
The use of ASCT for patients with relapsed/refractory MCL is not as common as in the frontline setting. The role of ASCT in first complete remission has been clearly shown to improve PFS in prior trials.[13] Current strategies to improve outcomes post ASCT have included rituximab maintenance, bortezomib maintenance, or treatment with a post-ASCT combination of rituximab and bortezomib to increase PFS. Graf et al reported a retrospective analysis at the Fred Hutchinson Cancer Research Center showing that post-transplant use of rituximab was associated with improved PFS (HR, 0.44 [CI, 0.24–0.80]; P = .007) and OS (HR, 0.46 [CI, 0.23–0.93]; P = .03).[38] At the 2015 ASH Annual Meeting, Kaplan et al reported on the use of consolidative bortezomib or maintenance bortezomib post ASCT.[39] In their prospective study, bortezomib was administered in a dose-intense manner for a shortened course, or in a less intense dose over a 2-year period; both treatment approaches were shown to improve PFS as compared with historical controls. At City of Hope, we are currently conducting a phase II multicenter trial using bortezomib plus rituximab in patients with MCL who are undergoing ASCT. Our interim analysis shows a 2-year disease-free survival of 100%, which is superior to that reported with historical controls.[40]
In the setting of relapsed disease, allogeneic stem cell transplantation (allo-SCT) is considered to be the only curative treatment. Tam et al reported using reduced-intensity conditioning (RIC) allo-SCT in patients with relapsed/refractory MCL.[41] With a median follow-up of 56 months, the 6-year PFS rate was 46% and the 6-year OS rate was 53%. Vaughn et al reported the use of a total body irradiation (TBI)-based nonmyeloablative regimen comprising 3 doses of fludarabine at 30 mg/m2 and low-dose (2 Gy) TBI as a conditioning regimen for patients with MCL. The results show a 5-year non-relapse mortality incidence of 28%, an OS rate of 55%, and a PFS rate of 46%. The 10-year OS rate was 44% and the 10-year PFS rate was 41%.[42] Although RIC allo-SCT can induce a long-term remission in patients with relapsed/refractory MCL, the risk of chronic graft-versus-host disease and infectious complications must be considered before initiating allo-SCT.
Monitoring Minimal Residual Disease (MRD)
Assessment of MRD has gained significant traction in the evaluation of treatment response among patients with lymphoid malignancies.[43,44] In acute lymphoblastic leukemia, MRD has been shown to be an independent predictive factor in determining long-term outcomes.[45,46] Several techniques can be used to assess patients for MRD. Real-time quantitative polymerase chain reaction (PCR) analysis of junctional regions of rearranged immunoglobulin heavy chain (IgH) genes is a highly sensitive method. Multicolor flow cytometry can also detect MRD at a high frequency. Bottcher et al showed that PCR was more sensitive at detecting MRD in MCL, compared with use of four-color flow cytometry. They found that 18% of patients tested were negative for MRD by flow cytometry but positive for MRD by consensus IgH/PCR.[47] More recently, a novel method of next-generation sequencing (NGS) can identify clonogenic B cells with high sensitivity and specificity. The novel method can overcome disadvantages of PCR-based methods and avoid the need for patient-specific agents. It also has the potential to operate at a higher level of sensitivity (sensitivity of 1 × 106), which is superior to flow cytometry. Genomic DNA is amplified using locus-specific primer sets of IgH (targeting the VDJ region) and immunoglobulin kappa locus (IGK) regions in MCL patients. The amplified product is then sequenced, and the sequences and frequencies of the clones are obtained. The cancer-derived sequences are then used as targets that assess for the presence of MRD in follow-up samples.[48,49] Recent studies have mainly used the NGS method as performed by Adaptive Biotechnologies. A recent SWOG study showed that RB achieved MRD-negative status for 89% of patients prior to transplant, and all patients who became MRD-negative had a 2-year PFS rate of 100%.[19]
Conclusion
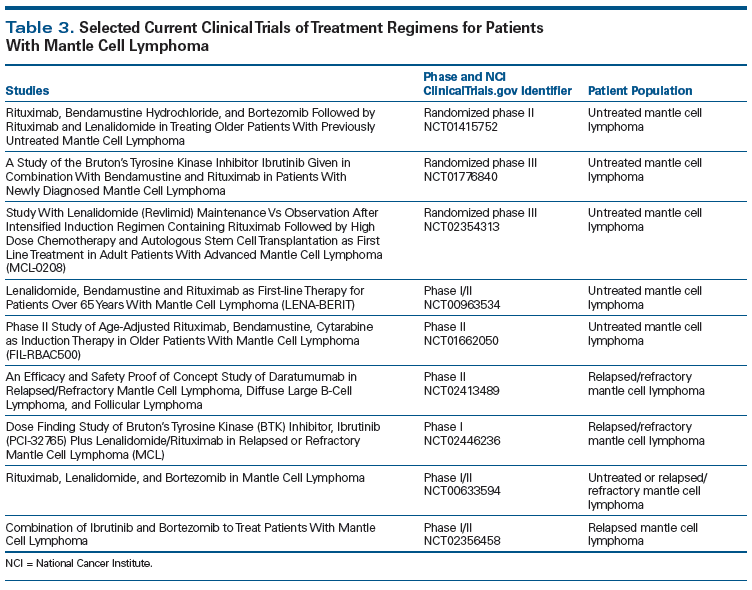
Significant progress has been made in understanding the molecular biology of MCL. These discoveries have been translated into clinical medicine in terms of better prognostic factors, improved methods of monitoring MRD, and novel therapeutics. The median OS of patients with MCL has been extended from 2.7 years to 4.8 years from the time period of 1975–1996 to 1996–2004.[50] Most of the novel therapeutics now in use or under investigation for MCL were discovered since 2004 and will certainly extend the median OS even further. Questions that remain to be answered include those related to identifying the optimal induction regimen, the role of ASCT in MRD-negative patients post induction, optimal maintenance therapy post induction, and optimal combinations of novel therapeutics. Table 3 provides a list of upcoming studies that may answer these questions; however, it is not a comprehensive list, since there are more trials yet to come. Fortunately, significant progress has been made in the treatment of MCL, with compelling prospects for further advances.
Financial Disclosure: Dr. Chen has received honoraria and speakers bureau payments from Genentech; consultancy and speakers bureau payments, as well as research funding, from Millennium; and research funding from Pharmacyclics. The other authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909-18.
2. Samaha H, Dumontet C, Ketterer N, et al. Mantle cell lymphoma: a retrospective study of 121 cases. Leukemia. 1998;12:1281-7.
3. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed, vol 2. Geneva, Switzerland: WHO Press; 2008.
4. Bertoni F, Rinaldi A, Zucca E, Cavalli F. Update on the molecular biology of mantle cell lymphoma. Hematol Oncol. 2006;24:22-7.
5. Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185-97.
6. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558-65.
7. Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010;115:1530-3.
8. Fernandez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70:1408-18.
9. Bernard M, Gressin R, Lefrere F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15:1785-91.
10. Hartmann E, Fernandez V, Moreno V, et al. Five-gene model to predict survival in mantle-cell lymphoma using frozen or formalin-fixed, paraffin-embedded tissue. J Clin Oncol. 2008;26:4966-72.
11. Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood. 2007;109:4599-606.
12. Chen RW, Bemis LT, Amato CM, et al. Truncation in CCND1 mRNA alters miR-16-1 regulation in mantle cell lymphoma. Blood. 2008;112:822-9.
13. Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677-84.
14. Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013-23.
15. Bernstein SH, Epner E, Unger JM, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol. 2013;24:1587-93.
16. Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687-93.
17. Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48-53.
18. Chen R, Li H, Bernstein S. Results of a randomized phase II trial of R-HCVAD vs R-bendamustine followed by autologous stem cell transplants for patients with mantle cell lymphoma: US Intergroup S1106. Presented at the 13th International Conference on Malignant Lymphoma; June 17-20, 2015; Lugano, Switzerland. Abstract 62.
19. Chen R, Li H, Bernstein S, Rimsza L. Pre-transplant R-bendamustine induces high rates of minimal residual disease in MCL patients: updated results of S1106: US Intergroup Study of a randomized phase II trial of R-HCVAD vs. R-bendamustine followed by autologous stem cell transplants for patients with mantle cell lymphoma. Blood. 2015;126:abstr 518.
20. Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288-94.
21. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520-31.
22. Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372:944-53.
23. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203-10.
24. Vidal L, Gafter-Gvili A, Dreyling M, et al. Rituximab maintenance (MR) for patients with mantle cell lymphoma (MCL) - a systematic review and meta-analysis of randomized controlled trials (RCTs). Blood. 2014;124:abstr 4466.
25. Ruan J, Martin P, Shah B, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373:1835-44.
26. Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209-13.
27. Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383-9.
28. Visco C, Finotto S, Zambello R, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol. 2013;31:1442-9.
29. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867-74.
30. Ansell SM, Inwards DJ, Rowland KM Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508-14.
31. Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24:2892-7.
32. Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13:716-23.
33. de Claro RA, McGinn KM, Verdun N, et al. FDA approval: ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3586-90.
34. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507-16.
35. Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739-45.
36. Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3K delta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123:3398-405.
37. Gerecitano JF, Roberts AW, Seymour JF, et al. A phase 1 study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;126:abstr 254.
38. Graf SA, Stevenson PA, Holmberg LA, et al. Maintenance rituximab after autologous stem cell transplantation in patients with mantle cell lymphoma. Ann Oncol. 2015;26:2323-8.
39. Kaplan L, Jung S, Stock W, et al. Bortezomib maintenance (BM) versus consolidation (BC) following aggressive immunochemotherapy and autologous stem cell transplant (ASCT) for untreated mantle cell lymphoma (MCL): CALGB (Alliance) 50403. Blood. 2015;126:abstr 337.
40. Chen R, Palmer J, Holmberg L, et al. Interim analysis of a phase 2 study of bortezomib plus rituximab maintenance therapy in patients with mantle cell lymphoma status post autologous stem cell transplantation. Blood. 2015;126:abstr 1961.
41. Tam CS, Bassett R, Ledesma C, et al. Mature results of the M.D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144-52.
42. Vaughn JE, Sorror ML, Storer BE, et al. Long-term sustained disease control in patients with mantle cell lymphoma with or without active disease after treatment with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Cancer. 2015;121:3709-16.
43. Pott C. Minimal residual disease detection in mantle cell lymphoma: technical aspects and clinical relevance. Semin Hematol. 2011;48:172-84.
44. Ferrero S, Drandi D, Mantoan B, et al. Minimal residual disease detection in lymphoma and multiple myeloma: impact on therapeutic paradigms. Hematol Oncol. 2011;29:167-76.
45. Stow P, Key L, Chen X, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115:4657-63.
46. Raff T, Gokbuget N, Luschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109:910-5.
47. Bottcher S, Ritgen M, Buske S, et al. Minimal residual disease detection in mantle cell lymphoma: methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica. 2008;93:551-9.
48. Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:5173-80.
49. Ladetto M, Bruggemann M, Monitillo L, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2014;28:1299-307.
50. Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511-8.