The immune checkpoint inhibitors ipilimumab, nivolumab, and pembrolizumab have dramatically improved outcomes for patients with metastatic melanoma; however, not all patients benefit from monotherapy with these agents. To address this issue, complementary combinations of immunotherapy are increasingly being explored as a strategy to improve outcomes. However, combinatorial approaches come with heightened risk of toxicity. In this review, we highlight combinations for which there are prospective data from clinical trials. The combinations discussed include ipilimumab plus anti–programmed death 1 agents, ipilimumab plus granulocyte-macrophage colony-stimulating factor, checkpoint inhibitor plus talimogene laherparepvec, ipilimumab plus chemotherapy, checkpoint inhibitor plus BRAF/MEK targeted therapy, and checkpoint inhibition plus radiation therapy. We discuss data regarding the efficacy and toxicity of combination therapy, and we identify clinical scenarios that may favor treatment with combination therapy.
Background
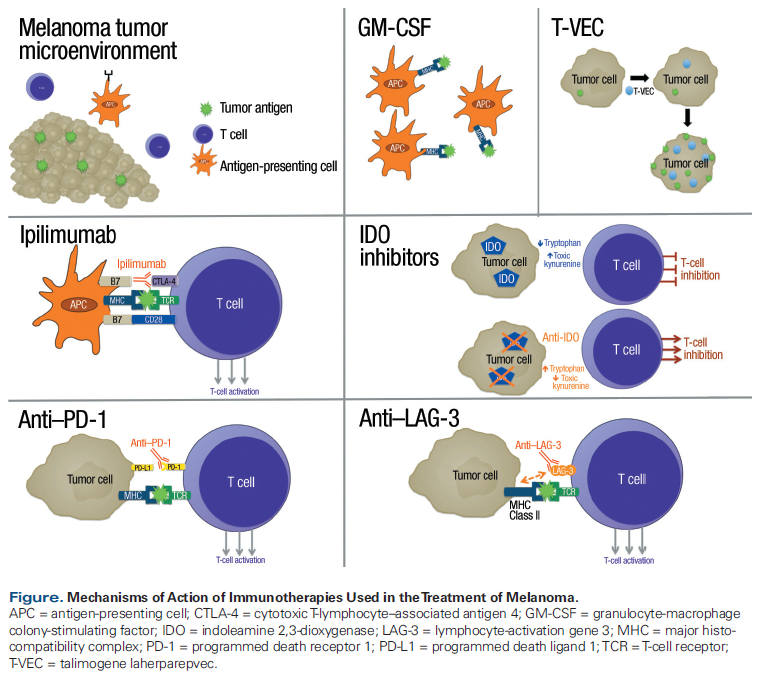
The immune system can eliminate cancer, so strategies to increase antitumor immunity have emerged as a primary therapeutic approach for the treatment of melanoma. While the immune system is complex and multifaceted, therapeutic approaches to antitumor immunity have largely focused on T cells. T-cell immunity is controlled by immune checkpoints. These are physiologic on/off switches that enable immune responses to potentially threatening antigens, while protecting the host from overzealous immune responses and autoimmunity.[1,2] For the treatment of melanoma, there are currently three checkpoint inhibitors that have been approved by the US Food and Drug Administration (FDA): ipilimumab, nivolumab, and pembrolizumab. These have two main targets: cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), which is targeted by ipilimumab, and programmed death receptor 1 (PD-1), which is targeted by nivolumab and pembrolizumab. All three of the approved checkpoint inhibitors are widely used in clinical practice.
Single-agent immune checkpoint inhibition has proved to be a successful strategy for the treatment of metastatic melanoma. Ipilimumab is also approved as adjuvant therapy for resected stage III disease. More recently, nivolumab was approved for adjuvant therapy based on an improvement in recurrence-free survival compared with ipilimumab in patients with resected stage IIIB, IIIC, or IV melanoma.
Despite the known efficacy of these agents, not all patients benefit from single-agent immune checkpoint inhibitor therapy, prompting further investigation into whether combining immune checkpoint inhibitors, either with each other or with other anticancer treatments, could improve outcomes. Combinations ideally should have complementary and not overlapping mechanisms of immune activation to maximize benefit and minimize toxicity.[3,4]
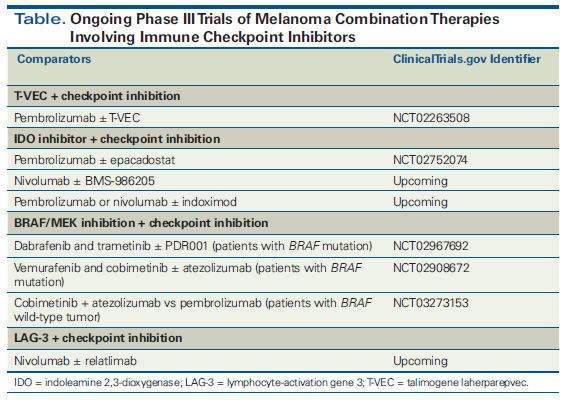
While combinatorial approaches hold considerable promise, it is important that the potential added risk of toxicity with a proposed combination therapy not outweigh the added benefit.[5] In this article, we take a practical approach to understanding the role of combination strategies for checkpoint inhibition in melanoma by examining data on the superiority of combinations vs single agents, and thereby trying to answer controversial questions of “which is better.” We focus on clinically relevant therapeutic strategies for which clinical trial data are available. A number of other combinations are being actively investigated-such as those that involve indoleamine 2,3-dioxygenase inhibitors or lymphocyte-activation gene 3-but these are not yet ready for the clinic due to the paucity of randomized data. The Figure shows the mechanisms of action of the various approaches we discuss. The Table lists ongoing phase III clinical trials of combinations of immune checkpoint inhibition with other approaches. In the many areas where much remains unknown, we highlight key questions in the field and propose strategies for remedying deficiencies in available knowledge.
Ipilimumab Plus Anti–PD-1 vs Ipilimumab Alone vs Anti–PD-1 Alone
CTLA-4 was the first immune checkpoint targeted for clinical intervention. CTLA-4 is expressed on the surface of T cells and regulates early activation, known as priming, of naive T cells during their first encounter with a tumor peptide, which is presented as part of the major histocompatibility complex by antigen-presenting cells in the draining lymph node.[6] Binding of CTLA-4 to its ligands on antigen-presenting cells inhibits T cells, so preventing this binding is one mechanism by which CTLA-4 blockade increases T-cell responses against tumors.[7] Ultimately, the anti–CTLA-4 monoclonal antibody ipilimumab was shown to improve overall survival (OS) in patients with advanced melanoma,[8] and ipilimumab was approved by the FDA for the treatment of metastatic melanoma in March 2011.
While CTLA-4 blockade functions primarily, though not exclusively, in lymph nodes, PD-1 is thought to operate primarily within the tumor microenvironment, where it decreases the activity of peripheral T cells. Therefore, anti–PD-1 therapy has a mechanism of therapeutic checkpoint inhibition that complements rather than overlaps that of CTLA-4 blockade. In a phase I clinical trial of the combination of ipilimumab and nivolumab at escalating doses in patients with melanoma, response rates were favorable compared with each agent as monotherapy. However, an increase in immune-related adverse events (irAEs) with the combination compared with each agent as monotherapy was also reported.[9,10]
The phase II CheckMate 069 study of 142 patients with advanced untreated melanoma, which compared ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg) vs ipilimumab alone, demonstrated an objective response rate (ORR) of 61% for combination therapy vs 11% for ipilimumab alone in patients with BRAF wild-type melanoma.[11] The risk of progression or death was reduced by 60% with combination therapy compared with ipilimumab alone (hazard ratio [HR], 0.40; 95% CI, 0.22–0.71; P < .002). In a subsequent exploratory OS analysis after a median of 24.5 months of follow-up, the 2-year OS rate in the ipilimumab + nivolumab arm was 63.8% (95% CI, 53.3%–72.6%) vs 53.6% in the ipilimumab-alone arm (95% CI, 38.1%–66.8%), and median OS was not yet reached in either arm.[12] Grade 3/4 toxicity was observed in 54% of patients treated with combination therapy and in 24% of patients treated with ipilimumab alone.
After the superiority of the combination over ipilimumab in short-term endpoints (response rates and progression-free survival [PFS]) had been demonstrated in CheckMate 069, the phase III trial (CheckMate 067) randomized 945 patients with advanced untreated melanoma to ipilimumab alone, nivolumab alone, or concurrent ipilimumab and nivolumab.[13] ORRs for the ipilimumab, nivolumab, and combination arms were 19%, 44%, and 58%, respectively. An updated survival analysis was recently published, with a minimum of 36 months of follow-up; this showed that the median OS was 19.9 months for ipilimumab, 37.6 months for nivolumab, and not yet reached for the ipilimumab + nivolumab arm (HR for death with ipilimumab plus nivolumab vs ipilimumab alone, 0.55; P < .001).[14] Three-year OS rates for ipilimumab, nivolumab, and combination therapy were 34%, 52%, and 59%, respectively. The study was not powered sufficiently to compare nivolumab alone against ipilimumab plus nivolumab.
Again, this study demonstrated that improved efficacy with combination therapy compared with ipilimumab alone comes with the detriment of higher toxicity. Grade 3/4 toxicity rates were 28.3% for ipilimumab, 16.3% for nivolumab, and 55% for combination therapy.[13] While 31% of patients receiving ipilimumab plus nivolumab stopped therapy because of adverse events (compared with 14.1% in the ipilimumab arm and 7.7% in the nivolumab arm), the ORR was 70% among those who discontinued therapy and median OS was not yet reached, raising questions about the optimal duration of combination immunotherapy.[15]
Given the impressive efficacy yet ongoing concerns about increased toxicity with the combination of checkpoint inhibitors targeting CTLA-4 and PD-1, there is considerable interest in altering schedules and/or dosing of CTLA-4 blockade to try to minimize irAEs while maintaining efficacy.[16,17] Specifically, altering the administration of ipilimumab by lowering the dose to 1 mg/kg in combination with anti–PD-1 therapy, or administering ipilimumab less frequently (ie, every 6 or 12 weeks), have been appealing strategies. In one single-arm nonrandomized study of reduced-dose ipilimumab (1 mg/kg) with standard-dose pembrolizumab, the response rate appeared similar to that seen with standard-dose ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg). However, ipilimumab is known to have dose-dependent effects on OS that are not necessarily dependent on response rates, given that 10 mg/kg of ipilimumab was recently shown to improve OS compared with 3 mg/kg, without an apparent difference in response rate or PFS.[18] Thus, clinicians must be cautious when considering reduced dosing of ipilimumab in combination with an anti–PD-1 agent based on response rate endpoints alone. Randomized trials with longer-term follow-up are needed to assess the true efficacy of these modifications.[19]
In summary, ipilimumab plus nivolumab is clearly superior to ipilimumab alone, but the question of whether combination ipilimumab plus nivolumab is superior to nivolumab alone remains a difficult dilemma for clinicians who must decide between these treatments for their patients. The exploratory data that demonstrate the highest efficacy for the combination are intriguing, but this advantage comes at the cost of higher toxicity, requiring appropriate patient selection and counseling.
KEY POINTS
- Ideal combinations should have complementary-not overlapping- mechanisms of immune activation to maximize benefit and minimize toxicity.
- Ipilimumab + nivolumab is clearly superior to ipilimumab alone, but the superiority of the combination compared with nivolumab alone is not clearly established. The risk of toxicity is significantly higher with combination therapy. We favor using ipilimumab + nivolumab in patients with multiple brain metastases who are not candidates for stereotactic radiosurgery, patients with mucosal melanoma, and those patients who are well enough.
- At this time, there are insufficient data to recommend the combination of checkpoint inhibitors + BRAF/MEK inhibition.
- While there is not clear evidence for improved efficacy with radiation therapy + checkpoint blockade, data suggest that the combination is safe, so concurrent treatment is reasonable to pursue when both modalities are independently indicated.
Unfortunately, no biomarker has been shown to be specifically predictive of treatment benefit from nivolumab plus ipilimumab compared with nivolumab (or pembrolizumab) alone. Nonetheless, testing for tumor programmed death ligand 1 (PD-L1) expression and mutational burden have been explored as potential predictive indicators of response to immunotherapy-with PD-L1 positivity and high mutational burdens being favorable for better outcomes.[20] Despite some exploratory signals that the relative benefits of nivolumab plus ipilimumab compared with nivolumab alone were greatest in patients who were defined as PD-L1–negative, a statistical test for interaction was not positive, suggesting that PD-L1 negativity is not statistically predictive of treatment benefit from combination immunotherapy compared with single-agent anti–PD-1 therapy.[13] Data for tumor mutational burden have not yet been reported in the setting of nivolumab plus ipilimumab. Therefore, while the hypotheses are provocative that perhaps patients with PD-L1–negative tumors or those with low mutational burden may derive greater benefit from nivolumab plus ipilimumab compared with single-agent anti–PD-1 therapy, the data are not strong enough to recommend using these markers to guide clinical practice.
Special considerations may be warranted for certain subgroups of patients. One example is patients with mucosal melanoma, who are generally believed to have a poor prognosis.[21] A pooled analysis of patients with mucosal melanoma (N = 86) who received ipilimumab plus nivolumab or nivolumab alone in a clinical trial was recently published.[22] Of these patients, the median PFS was 2.7 months in the ipilimumab arm, 3.0 months in the nivolumab arm, and 5.9 months in the combination arm. Combination immunotherapy response rates appeared lower in patients with mucosal melanoma (37%) than in patients with cutaneous melanoma (60%). Patients with mucosal melanoma had a response rate of 37% for combination immunotherapy vs a response rate of 23% for therapy with nivolumab alone. Notably, this absolute difference of 14 percentage points approximates the absolute difference in response rate between combination immunotherapy and nivolumab monotherapy seen in cutaneous melanoma. These data suggest that the choice of the combination of nivolumab plus ipilimumab for a patient with cutaneous melanoma should be largely based on the higher response rate and cannot simply be made based on the histology of the underlying melanoma subtype.
Similarly, particular attention has been paid to the efficacy of nivolumab plus ipilimumab in patients with brain metastases. At the American Society of Clinical Oncology (ASCO) 2017 Annual Meeting, data were presented from two phase II trials. One study consisted of 50 patients with asymptomatic melanoma brain metastases who had not received prior local brain therapy or immune checkpoint blockade.[23] Patients were randomized to ipilimumab plus nivolumab vs nivolumab alone. While not powered for a statistical comparison of OS, the 6-month OS rate was 59% for nivolumab alone vs 76% for ipilimumab plus nivolumab, suggesting an advantage to combination therapy for patients with intracranial metastases. Data from the CheckMate 204 study were also presented at the 2017 ASCO Annual Meeting; these demonstrated an intracranial ORR of 56% with the combination of ipilimumab plus nivolumab.[24] Although these are small studies, and longer-term follow-up with more patients is needed, these data suggest impressive efficacy for the combination of ipilimumab plus nivolumab in patients with brain metastases. In our practice, we generally prefer combination immunotherapy over single-agent anti–PD-1 therapy for patients with multiple brain metastases who are not candidates for stereotactic radiosurgery (SRS).
Most important to the choice of combination immunotherapy in any setting, however, is that patients are perceived to be capable of handling the possibility of irAEs and consequent treatment with corticosteroids. Patients who do not have supportive caregivers or who are perceived as having difficulty communicating with the care team may not be good candidates for combination immunotherapy, since management requires close communication and may necessitate adherence to immunosuppressive treatment regimens in the event of significant toxicity. Because the 3-year OS rates of nivolumab plus ipilimumab and nivolumab alone are similar, single-agent anti–PD-1 therapy remains an appropriate choice for many patients and is the standard control arm of most randomized clinical trials.
GM-CSF Plus Ipilimumab vs Ipilimumab Alone
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine naturally secreted by macrophages, T cells, mast cells, natural killer cells, endothelial cells, and fibroblasts to stimulate stem cells to produce granulocytes and monocytes. The monocytes subsequently mature into macrophages and dendritic cells. From an oncologic perspective, GM-CSF augments the ability of dendritic cells to present antigen to lymphocytes, thereby boosting the antitumor activity of T- and B-lymphocytes.[25,26] Hodi and colleagues conducted a phase II randomized clinical trial in 245 patients who had advanced melanoma that had progressed on at least one prior therapy. Patients were randomized to receive either ipilimumab (10 mg/kg) plus sargramostim (GM-CSF; 250 µg subcutaneously on days 1–4 of a 21-day cycle) or ipilimumab (10 mg/kg) alone.[27] At a median follow-up of 13.3 months, median OS in the ipilimumab + sargramostim group was 17.5 months (95% CI, 14.9 months–not reached) vs 12.7 months in the ipilimumab-alone arm (95% CI, 10.0 months–not reached; P = .01). Grade 3–5 toxicity was significantly lower in the ipilimumab + sargramostim arm than in the ipilimumab-alone arm (P = .04). Although follow-up time was short, this study provided the first evidence of an OS advantage with combination immunotherapy. The toxicity data are also reassuring. Despite the positive findings from incorporating GM-CSF into treatment with high-dose ipilimumab, this trial’s findings have not led to significant clinical use of GM-CSF with CTLA-4 blockade. Reasons for the limited uptake may be related to the difficulty of GM-CSF administration and the increase in use of the ipilimumab + anti–PD-1 combination at around the same time that the GM-CSF + ipilimumab combination data became available. Clearly, additional larger studies are needed with longer follow-up before conclusive recommendations can be made about the use of GM-CSF in addition to checkpoint blockade.
Checkpoint Inhibition Plus Talimogene Laherparepvec vs Checkpoint Inhibition Alone
Talimogene laherparepvec (T-VEC) is an oncolytic virus derived from herpes simplex virus type 1 that is FDA-approved for the treatment of advanced melanoma. It is the first oncolytic virus to produce meaningful clinical benefit in a randomized clinical trial for patients with solid tumors.[28] Administration of oncolytic viruses in patients with melanoma enhanced tumor antigen–specific T-cell response and abrogated the immunosuppressive function of regulatory T cells, suppressor CD8+ T cells, and myeloid-derived suppressor cells, providing a rationale for exploring T-VEC in combination with checkpoint inhibition.[29,30] Data were recently presented from an open-label, randomized phase II trial of 198 patients with advanced, unresectable stage IIIB–IV melanoma with injectable tumors who were given ipilimumab (3 mg/kg) with or without T-VEC.[31] The ORR was 38.8% for T-VEC plus ipilimumab vs 18.0% for ipilimumab alone (P = .002). In total, 28% of patients who received T-VEC plus ipilimumab and 18% of patients who received ipilimumab alone had grade 3/4 toxicity. These results are quite promising, and additional data are expected that will further inform decisions about this combination. Given the higher response rate and no obviously increased toxicity for the T-VEC + ipilimumab combination, for patients with unresectable or metastatic melanoma who have injectable lesions and for whom treatment with ipilimumab is appropriate, we favor combining ipilimumab with T-VEC, although we acknowledge this is not an approved combination.
T-VEC has also been studied in combination with anti–PD-1 therapy, including in a recently reported phase Ib trial with pembrolizumab that demonstrated a 62% confirmed response rate in patients with injectable melanoma lesions and advanced disease.[32] Despite this promise in a nonrandomized study, randomized data for T-VEC plus pembrolizumab are needed; a phase III randomized trial of pembrolizumab with or without T-VEC is ongoing (ClinicalTrials.gov identifier: NCT02263508).
Chemotherapy Plus Ipilimumab vs Ipilimumab Alone vs Chemotherapy Alone
Although traditionally viewed as immunosuppressive, chemotherapy can potentiate release of antigen and pro-inflammatory damage-associated molecular patterns, which in theory could be used to increase the efficacy of immune checkpoint blockade.[33] However, experience with this approach in melanoma has been limited to date. A randomized phase II trial of 72 patients with unresectable metastatic melanoma who had not received previous chemotherapy evaluated ipilimumab (3 mg/kg) plus dacarbazine (250 mg/m2/d) vs ipilimumab alone.[34] The combination arm had a higher ORR (14.3% vs 5.4%), but this difference was not statistically significant, probably because of the small number of patients in this study. Also, the response rate for the combination does not appear different from that seen with ipilimumab alone in other studies. To date, follow-up studies have not been conducted to determine whether adding chemotherapy to immune checkpoint blockade would be better than immune checkpoint blockade alone in melanoma.
A phase III trial of 502 patients randomized to higher-dose ipilimumab (10 mg/kg) plus dacarbazine (850 mg/m2) vs dacarbazine alone showed improved OS in the combination arm (11.2 months vs 9.1 months; HR, 0.72; P < .001).[35] As expected, grade 3/4 toxicity was increased in the combination arm (56.3% vs 27.5%; P < .001). The response rate in the ipilimumab + dacarbazine arm was 15.2%, similar to the published response rates for ipilimumab monotherapy. The trial did not compare the combination against ipilimumab alone. For this reason, and given the higher toxicity of this combination compared with ipilimumab monotherapy, this combination is not generally recommended for clinical use.
MAP Kinase Pathway–Directed Targeted Therapy Plus Immune Checkpoint Inhibition vs Targeted Therapy Alone
For melanoma that harbors a BRAF mutation, small molecules that target the combination of BRAF and MEK have become standard of care. FDA-approved BRAF inhibitors include dabrafenib and vemurafenib, and the approved MEK inhibitors are trametinib and cobimetinib. One of the tenets of combination strategies is that each agent should address a separate mechanism of antitumor action. Thus, combining BRAF/MEK inhibition with immunotherapy is an intriguing approach to improving efficacy and durability of response. Additionally, preclinical experiments indicated that treatment with BRAF inhibitors could increase antigen presentation and T-cell activation and proliferation.[36,37] Inhibition of CTLA-4 enhanced this effect in preclinical models.[38] However, both strategies can cause dermatologic and hepatic toxicities.
Unfortunately, the first report of such a combination, a phase I study of vemurafenib (960 mg orally twice daily) plus concurrent ipilimumab (3 mg/kg) in patients with metastatic BRAF V600–mutated melanoma, showed significant dose-limiting hepatotoxicity as well as rash.[39] These effects persisted at a lower dose of vemurafenib (720 mg twice daily), and thus the trial was closed to accrual. It is noteworthy that all aminotransferase levels were reversible with discontinuation of the agents and/or treatment with glucocorticoids. Sequential dosing, with administration of ipilimumab followed by vemurafenib, also caused severe skin toxicity, providing additional evidence of increased toxicity when these agents are given in proximity.[40] In a subsequent study, triple therapy with ipilimumab plus dabrafenib plus trametinib resulted in colitis followed by intestinal perforation in 2 of 7 patients.[41] The results of these studies have diminished enthusiasm for combining ipilimumab with MAP kinase pathway inhibitors.
PD-1/PD-L1 inhibitors are likely to be better combinatorial partners with BRAF and MEK inhibitors. At the 2017 ASCO Annual Meeting, data were presented on the combination of the PD-L1 inhibitor atezolizumab plus cobimetinib plus vemurafenib in BRAF V600–mutant metastatic melanoma.[42] Of 34 treated patients, 15 (44.1%) had grade 3/4 adverse events; 3 had to discontinue treatment because of transaminitis, and 1 patient discontinued treatment because of rash. At the time of the meeting, the ORR was 85.3%. While these data are promising, high response rates can be seen with the combination of BRAF + MEK inhibitors without immunotherapy, and the true question remains how much the addition of anti–PD-1/PD-L1 immunotherapy increases the duration of response. Clearly, data from ongoing randomized studies with longer follow-up are needed to determine the safety and efficacy of these combinations.
Concurrent Immune Checkpoint Inhibition Plus Radiation Therapy vs Immune Checkpoint Inhibition Alone
In addition to the systemic therapies discussed previously, radiation therapy (RT) is a mainstay of treatment for metastatic melanoma. Nearly half of patients with metastatic melanoma receive RT during their treatment, with palliative intent-to either relieve symptoms or prevent more serious complications (eg, impending cord compression, enlarging intracranial metastases). Because increasing numbers of patients are receiving immunotherapy, it is critical to understand the interactions between immune-based approaches and RT.
The role of RT in the era of immunotherapy for melanoma continues to evolve. For decades, scientists in the field of radiation biology have studied the abscopal effect, in which systemic effects in the nonirradiated field are observed following treatment with local RT. While radiation is generally thought to be immunosuppressive as a result of toxic effects on hematopoietic cells, studies have demonstrated that high-dose radiation causes tumor cell necrosis, releasing tumor-associated antigens that potentiate a systemic immune response.[43] Additionally, radiation has been shown to increase CD8+ T-cell infiltration, increase antigen presentation to dendritic cells, and promote pro-inflammatory cytokine signaling.[44,45] The mechanistic details of the interaction between RT and systemic immunity are reviewed elsewhere, and there is preclinical evidence to support the combination of RT with immunotherapy.[46,47] Here, we focus on available clinical data in melanoma.
Extracranial irradiation
The first prospective trial to evaluate checkpoint inhibition plus RT was a phase I trial of 22 patients with metastatic melanoma who were treated with RT targeting bone, liver, lung, and subcutaneous metastases, followed by ipilimumab (3 mg/kg).[48] Response rates and toxicity were similar to those seen in historical controls treated with ipilimumab monotherapy, suggesting that RT did not enhance the efficacy of ipilimumab in this setting.
In a separate pilot trial of 22 patients with metastatic melanoma, another group of investigators treated patients with ipilimumab (3 mg/kg) followed by RT within 5 days of the first dose of ipilimumab.[49] The median OS in this trial was 13.8 months, and nonirradiated tumor shrinkage was noted in 6 patients. Whether the shrinkage of nonirradiated tumors was purely attributable to ipilimumab alone (without RT) remains unknown, as nonrandomized studies are unable to assess for abscopal responses. Again, no added toxicity was seen above that expected from the individual therapies. To date, there has not been a randomized trial of ipilimumab with or without RT to definitively address whether the combination is superior to ipilimumab alone. We therefore cannot support adding RT to ipilimumab purely in hopes of eliciting an abscopal response. However, if a patient needs RT anyway, it appears safe to add RT to ipilimumab.
Anti–PD-1 therapy in combination with RT is also being explored. To date, no prospective clinical trial data are available. There are many ongoing clinical studies testing the combination of RT plus anti–PD-1 therapy (with or without additional ipilimumab; eg, ClinicalTrials.gov identifier: NCT02659540). At this time, there still are no prospective data that suggest adding RT to immune checkpoint inhibition improves the efficacy of checkpoint inhibition alone.
Intracranial irradiation
Over half of all patients with metastatic melanoma will develop brain metastases, and the majority of these metastases are often treated with SRS. Whether SRS enhances the efficacy of immune checkpoint inhibition for brain metastases is therefore an active area of interest. Since immunotherapy has efficacy in this patient population, there is controversy about adding SRS to the treatment regimen in these patients, making studies of SRS and immunotherapy especially important. Reports of combinations of immunotherapy with SRS are increasing, and the toxicity level is generally believed to be acceptable.[46] One group of investigators has reported biopsy-confirmed symptomatic necrosis of the brain following SRS and ipilimumab, but conclusions cannot be drawn from isolated case reports.[50]
Some retrospective analyses of patients who received the combination of ipilimumab plus SRS show improvements in OS with combination therapy compared with SRS alone, but others have not demonstrated the same improvement.[51-53] Additionally, in the patients with improved survival, it is unclear whether this improvement is simply a byproduct of the efficacy of systemic immunotherapy or a result of the combination approach.[54] In our practice, given safety data that generally indicate that SRS with immunotherapy has an acceptable toxicity profile, if a patient needs SRS and immunotherapy, we feel both are reasonable to pursue.
Conclusions
Advances in immunotherapy and the approval of immune checkpoint inhibitors have revolutionized the treatment of metastatic melanoma, but not all patients benefit from monotherapy with an immune checkpoint inhibitor. To overcome this, complementary combinations of immunotherapy are increasingly being explored as a strategy to improve outcomes. We have highlighted the combination strategies that have been studied in prospective trials and have attempted to identify clinical scenarios that would favor treatment with combination therapy. However, with so many potential combinatorial strategies, identifying optimal approaches and obtaining randomized data are paramount to maximize benefit and minimize toxicity. To maximize efficiency, cost-effectiveness, and potential benefit to patients, novel clinical trial designs are needed to explore the growing array of new immunotherapy combinations.
Financial Disclosure:Dr. Postow receives honoraria from Bristol-Myers Squibb and Merck; he serves on advisory boards for Array Biopharma, Incyte, Merck, New Link Genetics, and Novartis. Dr. Betof Warner has no significant financial interest in or other relationship with the manufacturer of any product mentioned in this article.
Acknowledgment: Dr. Betof Warner’s and Dr. Postow’s research has been funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
References:
1. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205-14.
2. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467-77.
3. Melero I, Berman DM, Aznar MA, et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457-72.
4. Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy. 2016;8:821-37.
5. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473-86.
6. Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852-63.
7. Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41-53.
8. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
9. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-33.
10. Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol. 2014;32(18 suppl):abstr LBA9003.
11. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-17.
12. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-68.
13. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
14. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-56.
15. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807-14.
16. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31-41.
17. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
18. Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611-22.
19. Postow M. Reduced-dose ipilimumab with standard-dose pembrolizumab: is less more? Lancet Oncol. 2017;18:1144-5.
20. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542-e551.
21. Kuk D, Shoushtari AN, Barker CA, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist. 2016;21:848-54.
22. D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226-35.
23. Long GV, Atkinson V, Menzies AM, et al. A randomized phase II study of nivolumab or nivolumab combined with ipilimumab in patients (pts) with melanoma brain metastases (mets): the Anti-PD1 Brain Collaboration (ABC). J Clin Oncol. 2017;35(15 suppl):abstr 9508.
24. Tawbi HA-H, Forsyth PAJ, Algazi AP, et al. Efficacy and safety of nivolumab (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the brain: results of the phase II study CheckMate 204. J Clin Oncol. 2017;35(15 suppl):abstr 9507.
25. Fischer HG, Frosch S, Reske K, Reske-Kunz AB. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882-8.
26. Weisbart RH, Golde DW, Clark SC, et al. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314:361-3.
27. Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312:1744-53.
28. Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780-8.
29. Kaufman HL, DeRaffele G, Divito J, et al. A phase I trial of intralesional rV-Tricom vaccine in the treatment of malignant melanoma. Hum Gene Ther. 2001;12:1459-80.
30. Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718-30.
31. Chesney JA, Puzanov I, Ross MI, et al. Primary results from a randomized (1:1), open-label phase II study of talimogene laherparepvec (T) and ipilimumab (I) vs I alone in unresected stage IIIB- IV melanoma. J Clin Oncol. 2017;35(suppl):abstr 9509.
32. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109-19.
33. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74-88.
34. Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29:489-98.
35. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-26.
36. Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213-9.
37. Koya RC, Mok S, Otte N, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012;72:3928-37.
38. Vella LJ, Pasam A, Dimopoulos N, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol Res. 2014;2:351-60.
39. Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365-6.
40. Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366:866-8.
41. Minor DR, Puzanov I, Callahan MK, et al. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res. 2015;28:611-2.
42. Sullivan RJ, Gonzalez R, Lewis KD, et al. Atezolizumab (A) + cobimetinib (C) + vemurafenib (V) in BRAFV600-mutant metastatic melanoma (mel): updated safety and clinical activity. J Clin Oncol. 2017;35(15 suppl):abstr 3063.
43. Sologuren I, RodrÃguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 2014;3:18-31.
44. Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516-23.
45. Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132-9.
46. Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: from concept to the clinic. Cancer. 2016;122:1659-71.
47. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498-e509.
48. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373-7.
49. Hiniker SM, Reddy SA, Maecker HT, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016;96:578-88.
50. Du Four S, Hong A, Chan M, et al. Symptomatic histologically proven necrosis of brain following stereotactic radiation and ipilimumab in six lesions in four melanoma patients. Case Rep Oncol Med. 2014;2014:417913.
51. Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117:227-33.
52. Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23:191-5.
53. Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2:899-906.
54. Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92:368-75.