Pancreatic cancer is one of the most lethal solid tumors. The prognosis of metastatic pancreatic adenocarcinoma remains dismal, with a median survival of less than 1 year, due in large part to the fact that pancreatic adenocarcinoma is notoriously refractory to chemotherapy. However, there recently have been significant improvements in outcomes for patients with pancreatic adenocarcinoma: ongoing trials have shown promise, and these may lead to still further progress. Here we review the current treatment paradigms for metastatic disease, focusing on ways to ameliorate symptoms and lengthen survival. We then summarize recent advances in our understanding of the molecular and cellular aspects of pancreatic cancer. Finally, we outline new approaches currently under development for the treatment of metastatic disease, arising from our improved understanding of the genetic and nongenetic alterations within pancreatic cancer cells-and of interactions between cancer cells, the tumor microenvironment, and the immune system.
Overview
An estimated 48,960 people will be diagnosed with pancreatic cancer in the United States in 2015, and 40,560 are estimated to die of the disease, making it the fourth most deadly malignancy.[1] The incidence of pancreatic cancer is increasing, and pancreatic cancer will likely become the second leading cause of cancer-related death in the United States by 2020.[2] The most common form of pancreatic cancer is pancreatic ductal adenocarcinoma, risk factors for which include cigarette smoking, chronic pancreatitis, diabetes, and obesity.[3-6] Pancreatic adenocarcinoma is also associated with several genetic syndromes, including BRCA1/2 and PALB2 mutations, hereditary pancreatitis, hereditary nonpolyposis colorectal cancer (Lynch syndrome), and Peutz-Jeghers syndrome.[7-11] The only curative approach for patients with pancreatic adenocarcinoma is surgical resection, but unfortunately the vast majority of patients with pancreatic ductal adenocarcinoma (80% to 90%) have surgically inoperable disease, with 53% of patients having clinical or radiographic evidence of metastatic disease at diagnosis. The estimated 5-year survival rate for patients with metastatic disease is only 2.4%, and in fact, 80% of patients die within 2 years of diagnosis.[12] However, we are now seeing improvements in these outcomes. The median overall survival (OS), which was steady for years at about 6 months, is now routinely 8.5 to 11 months, with a much larger percentage of patients surviving a year or more.[13,14] Here we review the trials that have led to these improvements, thus establishing new expectations; we also explore exciting new approaches to the treatment of metastatic pancreatic adenocarcinoma.
Established and Modern Therapeutic Strategies
Systemic chemotherapy
Chemotherapy remains the mainstay of the treatment of metastatic pancreatic adenocarcinoma; importantly, chemotherapy has consistently been shown to improve both OS and quality of life (QOL). The improvement in QOL is important to emphasize to patients (and their referring physicians), as they are often skeptical about the benefits of chemotherapy. However, the reality is that the persistent and often severe symptoms of pancreatic cancer itself (eg, pain, fatigue, and anorexia) are often ameliorated by chemotherapy, even when the side effects of chemotherapy are taken into account.
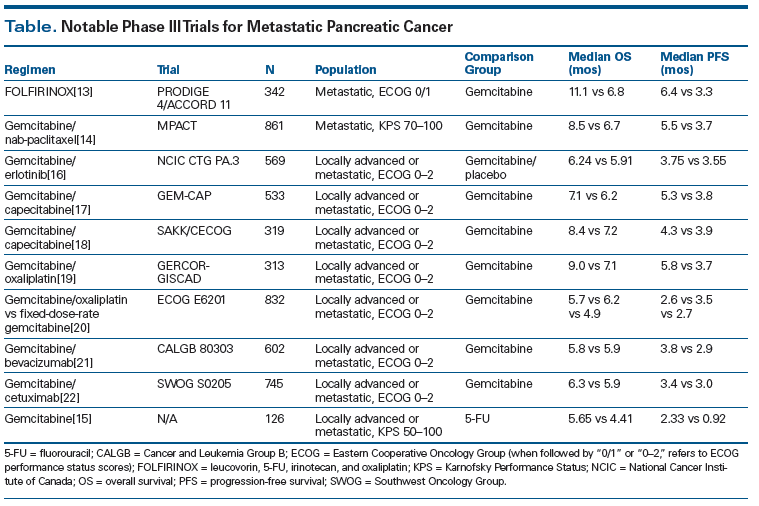
For nearly 15 years, the standard of care for patients with metastatic pancreatic adenocarcinoma was single-agent gemcitabine. Gemcitabine was first shown to be superior to fluorouracil (5-FU) in the advanced setting by Burris et al.[15] In a trial that randomized 126 patients in a 1:1 fashion either to gemcitabine (1,000 mg/m2 weekly for 7 weeks followed by 1 week of rest, then weekly for 3 weeks every 4 weeks thereafter) or to 5-FU (600 mg/m2 weekly), gemcitabine improved the median OS to 5.7 months, compared with only 4.4 months in the 5-FU arm (P = .0025). The median progression-free survival (PFS) was also improved-to 2.3 months with gemcitabine, compared with 0.9 months for 5-FU alone (P = .0002). Finally, gemcitabine improved QOL scores compared with 5-FU alone, and in fact, the original approval of gemcitabine by the US Food and Drug Administration (FDA) was based on this improvement in QOL.
For the subsequent 10 to 15 years, multiple phase III trials attempted to improve on the benefits observed with single-agent gemcitabine (Table). However, until recently, combination regimens involving gemcitabine failed to show a significant survival benefit. For example, the addition of erlotinib to gemcitabine compared with gemcitabine alone was demonstrated by Moore et al[16] to yield slight improvements in median PFS (3.75 vs 3.55 months; P = .004) and median OS (6.24 vs 5.91 months; P = .038). However, these marginal clinical benefits came at the cost of more toxicity (notably skin rash and an interstitial lung disease–like syndrome), and while the addition of erlotinib to gemcitabine was approved by the FDA, in practice the combination was not widely utilized.
There were a few phase III trials whose results were considered negative but which are interesting to reconsider, especially in light of the magnitude of the improvement in survival seen with gemcitabine plus nab-paclitaxel compared with gemcitabine alone (discussed below). For example, the combination of capecitabine plus gemcitabine compared with gemcitabine alone did result in an improvement in median PFS (5.3 vs 3.8 months; P = .004), but the improvement in median OS was not statistically significant (7.1 vs 6.2 months; P = .08).[17] In a comparable study by Herrmann et al,[18] patients with good performance status (PS) did see a significant benefit with the combination of capecitabine and gemcitabine, with an improvement in median OS to 10.1 months (compared with 7.4 months with gemcitabine alone). Another “negative” trial compared gemcitabine plus oxaliplatin vs gemcitabine alone.[19] In this trial of 326 patients, the median OS in the gemcitabine-plus-oxaliplatin arm was 9.0 months, compared with 7.1 months for gemcitabine alone (P = .13). However, the study was powered for an improvement of 8.0 vs 6.0 months, so the results were not statistically significant and the combination was not accepted as practice-changing. In addition, fixed-dose-rate (FDR) gemcitabine (1,500 mg/m2 over 150 minutes on days 1, 8, and 15 every 28 days) and gemcitabine plus oxaliplatin (gemcitabine, 1,000 mg/m2 over 100 minutes, on day 1 and oxaliplatin, 100 mg/m2 over 120 minutes, on day 2, every 14 days) were both noninferior to standard-dose gemcitabine in advanced disease in the Eastern Cooperative Oncology Group (ECOG) 6201 phase III trial (median OS of 6.2 months for FDR gemcitabine and 5.7 months for gemcitabine plus oxaliplatin, compared with 4.9 months for standard-dose gemcitabine; P = .04, .22, respectively, when compared with standard-dose gemcitabine [significance level was predefined as P < .025]).[20]
Other trials whose results were considered negative involved combinations using bevacizumab and cetuximab. In a phase III study (Cancer and Leukemia Group B [CALGB] 80303), Kindler et al demonstrated that the addition of bevacizumab (10 mg/kg on days 1 and 15) to gemcitabine in patients with advanced disease did not improve median OS compared with gemcitabine plus placebo (5.8 vs 5.9 months; P = .95).[21] The Southwest Oncology Group (SWOG) 0205 study of gemcitabine plus cetuximab (cetuximab loading dose of 400 mg/m2 over 120 minutes followed by weekly doses of 250 mg/m2 over 60 minutes) vs gemcitabine alone in patients with advanced disease also did not demonstrate a median OS benefit (6.3 vs 5.9 months; P = .23).[22] Surprisingly, no large phase III trials of gemcitabine plus either docetaxel or paclitaxel were ever pursued.
During the 2000s, another approach to improving outcomes that was tried was the three-drug cocktail. For example, the combination of gemcitabine with docetaxel and capecitabine (GTX) was studied in two retrospective analyses of patients with metastatic pancreatic adenocarcinoma. De Jesus-Acosta et al reviewed 154 patients treated in the frontline setting with GTX and found that the median OS was 11.6 months,[23]and Fine et al analyzed 35 patients (34% previously treated) and found a median OS of 11.2 months.[24] Finally, in a phase II trial of 46 patients with untreated advanced pancreatic adenocarcinoma, the three-drug combination of FOLFIRINOX (5-FU, leucovorin, oxaliplatin, and irinotecan) showed a very promising overall response rate (ORR) of 26% and a median OS of 10.1 months.[25]
Modern standards for metastatic disease
There are currently two “standard” options for the treatment of metastatic pancreatic adenocarcinoma-FOLFIRINOX or gemcitabine plus nab-paclitaxel (albumin-bound paclitaxel)-both of which were shown to be superior to gemcitabine alone in recent trials. In a study by Conroy et al, 342 patients were randomized to receive FOLFIRINOX (400 mg/m2 of bolus 5-FU, 400 mg/m2 of leucovorin, 85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan, and 2,400 mg/m2 of continuous infusion 5-FU over 46 hours-given every 2 weeks) vs single-agent gemcitabine (with the standard dosing of 1,000 mg/m2 weekly for 7 of 8 weeks, then weekly for 3 of 4 weeks).[13] The primary endpoint was median OS, and indeed FOLFIRINOX significantly improved median OS-to 11.1 months, compared with 6.6 months for gemcitabine alone (P < .001).[13] The median PFS was also significantly improved with FOLFIRINOX-to 6.4 months, compared with 3.3 months for gemcitabine alone (P < .001). This improvement came with increased toxicity, however, with statistically significant increases in neutropenia, febrile neutropenia, thrombocytopenia, alanine aminotransferase elevation, diarrhea, and neuropathy in the FOLFIRINOX arm. Patients in this study were also generally younger (median age, 61 years, with a maximum age of 75) and had good PS (38% had an ECOG PS of 0, and 62% had an ECOG PS of 1). Nevertheless, in a preplanned QOL analysis, patients who received FOLFIRINOX had a much lower rate of degradation of QOL at 6 months: 31%, compared with 66% with gemcitabine alone. The FOLFIRINOX regimen has frequently been adapted, with the resulting “modified” FOLFIRINOX (mFOLFIRINOX) regimens at least dropping the 5-FU bolus. Retrospective reviews[26] of mFOLFIRINOX have demonstrated median OS rates similar to those seen with the FOLFIRINOX regimen used by Conroy et al.[25]
More recently, gemcitabine plus nab-paclitaxel was shown by Von Hoff et al to increase both median PFS (5.5 vs 3.7 months; P < .001) and median OS (8.5 vs 7.6 months; P < .001) in metastatic pancreatic adenocarcinoma compared with gemcitabine alone.[14] This was a multi-institutional international trial of 861 patients that included patients with a worse PS than in the FOLFIRINOX trial (Karnofsky Performance Status [KPS] score of 70 or greater, roughly equivalent to an ECOG PS of 2 or lower), and there were no limitations on patient age. The combination of gemcitabine and nab-paclitaxel was well tolerated; the main adverse events were febrile neutropenia and peripheral neuropathy. Because the combination of gemcitabine and nab-paclitaxel has been well tolerated, it has formed the backbone of several ongoing trials hoping to further improve outcomes in patients with metastatic pancreatic adenocarcinoma through the addition of novel targeted agents.
National Comprehensive Cancer Network category 1 recommendations for first-line systemic therapy in patients with metastatic pancreatic adenocarcinoma and good PS include enrollment in a clinical trial; FOLFIRINOX or gemcitabine plus nab-paclitaxel (both preferred); or other options, including gemcitabine plus erlotinib and gemcitabine alone (preferred for patients with poor PS).[27] In these guidelines, good PS is defined as controlled pain, a patent biliary stent (if applicable), and good nutritional status.
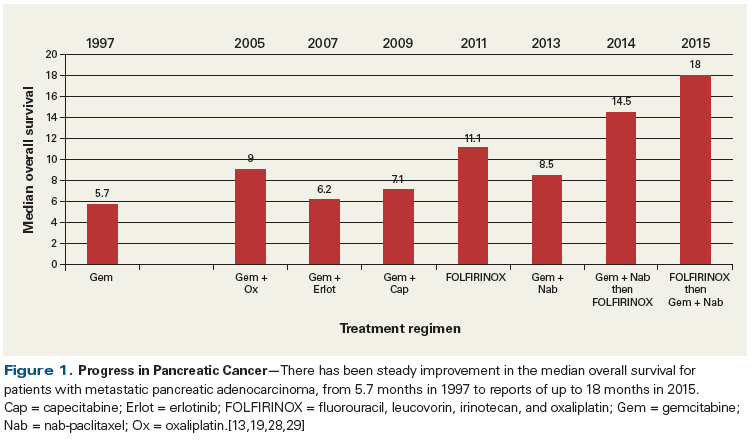
Finally, the concept of sequencing FOLFIRINOX and gemcitabine plus nab-paclitaxel is also likely to have a significant impact on the outcomes of patients with metastatic pancreatic adenocarcinoma. A pilot study by Ramanathan et al demonstrated that patients treated with 6 cycles of gemcitabine plus nab-paclitaxel followed by 6 cycles of FOLFIRINOX had a median OS of 14.5 months.[28] More recently, Portal et al examined OS as measured from the time of diagnosis in patients receiving second-line therapy with gemcitabine and nab-paclitaxel.[29] This was a prospective observational study of 57 patients who had received FOLFIRINOX in the frontline setting. Remarkably, the median OS from the time of diagnosis was 18.0 months, a stunning improvement for patients with metastatic pancreatic adenocarcinoma. Overall, in reviewing the progress of the last few years in metastatic pancreatic adenocarcinoma, the median OS is consistently improving, and great promise is being demonstrated as new therapies are incorporated (Figure 1).
Novel chemotherapeutic approaches
Many new therapies have emerged that offer promise for improving the treatment of metastatic pancreatic adenocarcinoma; among these are novel cytotoxic chemotherapies. For example, nanoliposomal encapsulated irinotecan (MM-398) is a novel chemotherapy formulation just approved by the FDA (October 22, 2015) for the treatment of metastatic pancreatic adenocarcinoma in the second-line setting. In a phase III trial, patients with metastatic pancreatic adenocarcinoma previously treated with gemcitabine were randomized 1:1:1 to receive MM-398 (120 mg/m2 over 90 minutes) every 3 weeks; 5-FU (2,000 mg/m2 over 24 hours) plus leucovorin (400 mg/m2 over 30 minutes) every 2 weeks; or a combination of MM-398 (80 mg/m2 over 90 minutes), 5-FU (2,400 mg/m2 over 46 hours), and leucovorin (400 mg/m2 over 30 minutes) every 2 weeks.[30] In the intent-to-treat population, median OS was significantly longer in the combination arm (6.1 months) compared with the 5-FU/leucovorin arm (4.2 months; P = .012). Grade 3 adverse events in the combination arm included neutropenia, fatigue, diarrhea, and vomiting. It is unclear whether MM-398 offers any benefit over standard irinotecan; however, this was the first randomized phase III metastatic pancreatic adenocarcinoma trial in the second-line setting to demonstrate a benefit in one arm.
TH-302, another novel agent, is a hypoxia-activated prodrug that in hypoxic settings releases the DNA alkylating agent bromo-isophosphoramide mustard. In an open-label phase II trial, patients with untreated advanced pancreatic cancer and an ECOG PS of 0/1 were randomized 1:1:1 to receive gemcitabine alone (1,000 mg/m2 over 30 minutes); gemcitabine plus TH-302 at 240 mg/m2; or gemcitabine plus TH-302 at 340 mg/m2. Treatment was given on days 1, 8, and 15 of a 28-day cycle.[31] Seventy-six percent of patients had metastatic pancreatic adenocarcinoma, while 24% had locally advanced, unresectable disease. Patients whose disease progressed on gemcitabine alone were allowed to cross over and were then randomized between the two TH-302 arms. Median OS was 9.2 months in the 340-mg/m2 arm (P = .39) and 8.7 months in the 240-mg/m2 arm (P = .77), compared with 6.9 months in the gemcitabine-alone arm. While the median OS differences were not statistically significant, median PFS was significantly longer in both TH-302 arms-6.0 months in the 340-mg/m2 arm (P = .008) and 5.6 months in the 240-mg/m2 arm (P = .040)-compared with the gemcitabine-alone arm, where median PFS was 3.6 months. In addition, median OS in the 26 crossover patients was significantly prolonged in those who were randomized to the 340 mg/m2-arm compared with those randomized to the 240 mg/m2-arm (12.2 vs 2.6 months; P = .004). Rash, stomatitis, and myelosuppression were more common in the TH-302 arms, but these adverse events did not lead to a higher rate of treatment discontinuation.
Cellular and Molecular Heterogeneity: Key Elements of Pancreatic Ductal Adenocarcinoma
Chief somatic mutations and therapies targeting DNA repair deficiencies
To date, hundreds of pancreatic ductal adenocarcinoma genomes have been sequenced in the hope of identifying gross and frequent genomic abnormalities and somatic (ie, tumor-specific) mutations within the coding regions of established cancer genes. In the early 1990s, a “gene jock” approach was taken, and as important functional genes or regions of the genome were discovered, investigators raced against one another to find the next frequently mutated tumor suppressor or oncogene. From this golden era of amplification of genomic regions and Sanger sequencing emerged a powerful understanding of the high-frequency somatic mutations that occur in pancreatic ductal adenocarcinoma and a pathologically based model of the progression of pancreatic adenocarcinoma from a gene-centric perspective.[32]
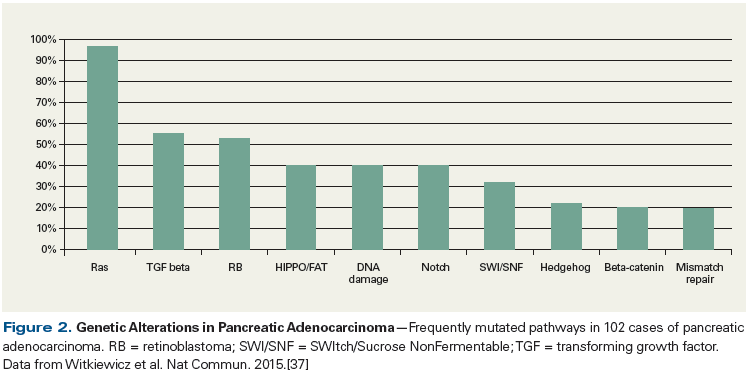
Arguably the most intriguing and robust finding of this era was that KRAS is mutated in more than 90% of pancreatic ductal adenocarcinomas and that this mutation occurs early in tumorigenesis (eg, it is seen in precursor lesions-pancreatic intraepithelial neoplasia, or PanIN).[33,34] From a theoretical standpoint, having an activated oncogene that occurs early and that plays a role in many aspects of tumorigenesis[35,36] provides a promising, active, druggable target, which many pharmaceutical companies, along with academic laboratories, have invested in. However, to date this knowledge has yielded little to no gain for any pancreatic cancer patient. Still, innovative strategies to target this frequently activated oncogene continue to be explored, and in fact, the National Cancer Institute has dedicated resources to a “Ras Initiative” (at the Frederick National Laboratory for Cancer Research) in an effort to aggressively invent strategies to target this pathway.
The above-mentioned work, along with complementary studies from other groups, has validated and discovered other rare actionable “needle in the haystack” mutations in various genes and pathways, including ERBB2, MET, FGFR1 (fibroblast growth factor receptor 1), Wnt signaling, chromatin remodeling, and Hedgehog (Hh) signaling (Figure 2).[37] Targeting a mutated pathway is logical, and many in the field are motivated to develop targeted therapeutic approaches, since detection of a mutation in the tumor that is not present in normal tissue provides an unquestionable therapeutic opportunity and a valued predictive biomarker. However, many additional factors are at play and suggest several reasons why targeting a single genetic lesion such as mutated KRAS has met with little success. These reasons include:
1) Genetic heterogeneity may be an issue (ie, does every cell in the tumor carry the same set of mutations, especially after treatments or when a clone becomes a distant metastatic lesion?).[38]
2) The mutation that has been selected for may be critical for a moment in time for that tumor (or clone), but by the time the tumor is treated, it may not be critical for the viability of the tumor cells.
3) Compensatory mechanisms may be at play (eg, Kras signaling).
Even though many of the frequently found mutations in pancreatic ductal carcinoma (ie, mutations in KRAS, TP53, and SMAD4) have driven focused and valued research in the field, it is perhaps the less frequently found mutations, such as those in the DNA repair pathway genes (BRCA2, PALB2, Fanconi anemia genes) that may be most actionable and have the most relevance (Figure 3). These mutations are thought to confer sensitivity to platinum-based chemotherapy and to poly (ADP-ribose) polymerase (PARP) inhibitors via synthetic lethality, the susceptibility to DNA damage due to a defect in a DNA repair pathway.[39] It is estimated that less than 5% of pancreatic ductal adenocarcinomas are characterized by defective BRCA2, and an additional small percentage are characterized by defects in the related PALB2 and Fanconi anemia genes.[40-42] Moreover, a recent next-generation sequencing study performed on multiple pancreatic ductal adenocarcinoma genomes estimated that a larger subpopulation of these cancers are defective in the DNA repair pathway, and in fact, the investigators defined a “mutational signature of DNA damage repair deficiency” that may be used to predict for susceptibility to platinum- and/or PARP inhibitor–based therapies.[43]
The use of PARP inhibitor–based therapies has demonstrated dramatic benefits for the small subgroup of patients with defects in the DNA repair pathway. Our institution is conducting an ongoing trial of the PARP inhibitor veliparib in combination with 5-FU and oxaliplatin, and in the phase I portion, patients with underlying BRCA2 mutations had OS durations of over 2 years, compared with only 8.6 months in the patients without BRCA mutations.[44] Moreover, in a phase II study, the oral PARP inhibitor olaparib showed clinical benefit in patients with a germline BRCA1/2 mutation and advanced pancreatic cancer who had progressed on gemcitabine, with a median PFS of 4.6 months and a median OS of 9.8 months.[45] The ongoing phase III POLO trial is investigating switch maintenance using olaparib in patients with stable metastatic pancreatic adenocarcinoma after a first-line platinum-based chemotherapy regimen.[46] The next few years should be an exciting time for the subgroup of patients with DNA repair defects, as presentations and manuscripts will be publicly disseminated about PARP inhibitor–based clinical trials in these patients.
Beyond genetics: targeting other key molecular drivers of metastatic pancreatic adenocarcinoma
Other mechanisms of gene regulation are also important to the survival of pancreatic ductal adenocarcinoma cells, yet their role is harder to prove and they are harder to target than a defined genetic mutation. Epigenetic modifications, transcriptional mechanisms, and post-transcriptional gene regulatory mechanisms have all been shown to be important in different pancreatic ductal adenocarcinoma models and patient samples. The clearest evidence that an epigenetic modification contributes to pancreatic tumorigenesis is the discovery that, with a genetic disruption in p16 (eg, loss of heterozygosity within the CDKN2A gene), one of the most common ways to silence p16 was methylation of CpG islands within the gene’s promoter region.[47] Aberrations in methylation patterns of key cancer genes, along with defects in other molecular mechanisms, such as chromatin remodeling,[48] may not only be valuable as markers for early detection but someday may be actionable targets in pancreatic ductal adenocarcinoma cells.
In addition, key cell signaling pathways have been shown to play a central role in the growth of pancreatic ductal adenocarcinoma cells, and targeting these pathways is showing promise for the treatment of metastatic pancreatic adenocarcinoma. For example, cyclin-dependent kinase (CDK) inhibitors are a group of novel targeted therapies currently under development. Because the CDKN2A gene (which encodes p16) is frequently mutated or lost in pancreatic cancer, the cells lose a potent inhibitor of CDK4/6 and thus lose a brake on cell-cycle progression.[49] While CDK4/6 inhibition alone has a limited effect on pancreatic tumor cells in vitro, due to upregulation of cyclin E, the addition of a mammalian target of rapamycin (mTOR) inhibitor or a MEK inhibitor has a synergistic effect, contributing to the halting of cell-cycle progression.[50] Clinical studies of CDK inhibitors in combination with other signaling pathway inhibitors in pancreatic cancer are ongoing.
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"43374","attributes":{"alt":"","class":"media-image","id":"media_crop_9757388559253","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"4725","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","title":" ","typeof":"foaf:Image"}}]]
Eileen M. O’Reilly, MD
David M. Rubenstein Center for Pancreatic Cancer Research, Memorial Sloan Kettering Cancer Center
Weill Cornell Medical College New York, New YorkPancreatic adenocarcinoma remains fundamentally challenging, although there have been tangible improvements in outcomes over the last couple of years with use of the modern standards of FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) and gemcitabine plus nab-paclitaxel for advanced disease, along with increasing integration of these regimens into earlier-stage disease settings. Weinberg and colleagues have provided a concise summary of current state-ofthe- art treatment, along with a comprehensive, insightful, and topical review of key novel therapeutics in development for pancreatic adenocarcinoma.What Are the Chief New Developments in Pancreatic Cancer Research?
The molecular genomics of the disease have been well depicted of late; there has also been a growing recognition that the epigenome and the tumor microenvironment are critical components that contribute to poor outcomes and that present opportunities for therapeutic exploitation. Strategies capitalizing on homologous repair deficiencies and stromal disruption strategies are highlighted. The prospect of immune therapeutics is on the horizon, and limited data thus far have suggested potential utility in pancreatic adenocarcinoma. As the authors note, the first forays with immune therapies have yielded minimal benefit; however, various combination immune strategies using vaccines, stromal modulating agents, checkpoint inhibitors, and chimeric antigen receptor (CAR) Tcells, all of which are under active investigation, hold promise.What Key Challenge Do Researchers Now Face?
Part of the challenge going forward will be to adopt innovative trial designs, in order to try to evaluate multiple strategies in parallel, as well as investigate possibilities for making informed prioritization decisions. In sum, progress in pancreatic adenocarcinoma has historically been slow, yet while current standard practice is not imminently changing, the explosion of development of novel therapeutics based on sound science, along with a resurgence of interest by the research and pharmaceutical communities, collectively position the field for progress.Financial Disclosure: Dr. O'Reilly consults for and receives research funding from Celgene. She has no other relevant disclosures.
The Janus kinase (JAK) pathway is another cell signaling target, and the JAK inhibitor ruxolitinib is an anti-inflammatory drug that acts on the JAK/STAT pathway. Patients with an elevated systemic inflammatory response to pancreatic cancer overall carry a worse prognosis; thus, studies have evaluated ways to reduce the inflammatory response in this subset of patients.[51] The RECAP study looked at second-line therapy with capecitabine plus ruxolitinib vs capecitabine plus placebo in patients with metastatic pancreatic adenocarcinoma. While there was no survival benefit overall, patients with an initial C-reactive protein (CRP) level above 13 mg/L did have an OS benefit with ruxolitinib as compared with placebo (hazard ratio [HR], 0.47 [95% confidence interval (CI), 0.26–0.85]; P = .01).[52] The ongoing JANUS 1 and JANUS 2 trials are evaluating capecitabine plus ruxolitinib vs capecitabine plus placebo as second-line therapy in patients with advanced disease and evidence of a systemic inflammatory response (a modified Glasgow Prognostic Score of 1 [CRP > 10 mg/L and albumin ≥ 35 g/L] or 2 [CRP > 10 mg/L and albumin < 35 g/L]).[53,54] The JAK1/2 inhibitor momelotinib is also being studied in a phase II trial of gemcitabine and nab-paclitaxel plus momelotinib vs gemcitabine and nab-paclitaxel plus placebo as first-line treatment of metastatic pancreatic adenocarcinoma.
Other less studied non–genetically based mechanisms include post-transcriptional gene regulation, guided primarily by micro RNAs (mRNAs)[55,56] and RNA-binding proteins. One example of the latter, the RNA-binding protein HuR, binds to a subset of survival mRNAs important for pancreatic tumorigenesis and is also important for keeping pancreatic ductal adenocarcinoma cells viable within the pancreatic adenocarcinoma microenvironment.[57-59] HuR can regulate important cell-cycle regulator molecules such as WEE1[57] when pancreatic adenocarcinoma cells are exposed to chemotherapeutic stress. The regulation of the mitotic kinase inhibitor WEE1 is a rapid and tractable method that pancreatic adenocarcinoma cells adopt in order to resist therapy. In fact, WEE1 is currently being explored as a target in pancreatic ductal adenocarcinoma in order to overcome G2/M arrest (chemotherapeutic resistance) that arises because of frequent mutations that affect the G1/S phase of the cell cycle (eg, within TP53).[60] Two other clinically relevant examples of how HuR helps pancreatic ductal adenocarcinoma cells survive within the tumor microenvironment are the ways in which HuR is activated under nutrient deprivation[61] and in the setting of hypoxia, through its regulation of PIM1 kinase.[62] Taken together with the observation that HuR is more abundant in tumor cells than in normal cells,[58,63,64] the data from these investigations support the notion that HuR is critical for pancreatic ductal adenocarcinoma cell development and survival within the tumor microenvironment and would make an attractive intact target in pancreatic adenocarcinoma. In fact, Jimbo et al recently provided proof-of-principle data that targeting HuR in preclinical mouse models of pancreatic ductal adenocarcinoma dramatically reduced tumor development and growth.[65] Moreover, a small-molecule inhibitor of HuR (MS-444)[66] is being tested in mice to evaluate this promising concept and to move this targeted strategy towards the clinic.
The cellular components of the tumor microenvironment
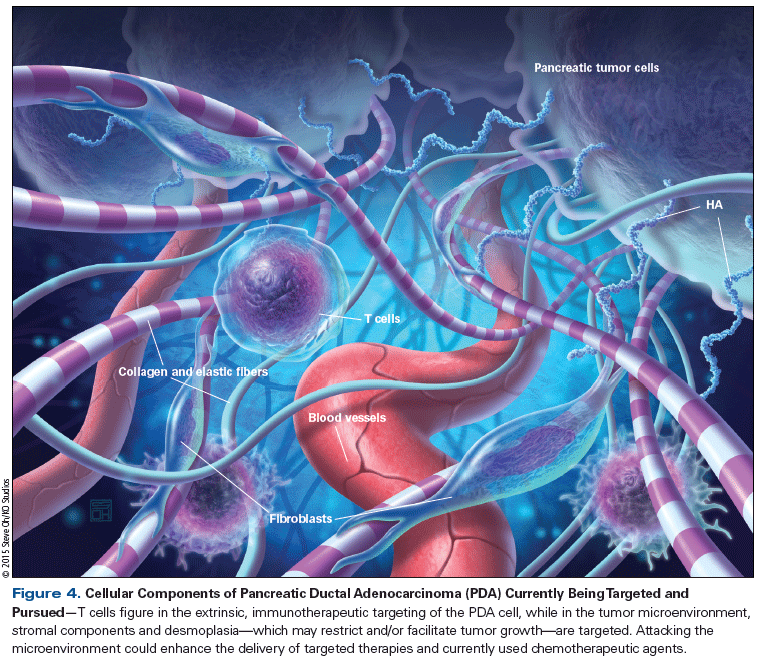
Pancreatic ductal adenocarcinoma has a diverse cellular population and is probably one of the most densely stromal embedded tumors. In fact, it is common for nontumor components (ie, vessels, stellate cells, myofibroblasts, and extracellular matrix) to outnumber tumor cells in any given pancreatic adenocarcinoma tumor mass. The components that make up the pancreatic ductal adenocarcinoma tumor microenvironment are: 1) always changing and appear to be important during the tumorigenesis process; 2) most likely play a role in pancreatic adenocarcinoma cell survival and resistance to specific therapies; and 3) may be a critical element to target in the pancreatic ductal adenocarcinoma tumor mass (Figure 4).[67] Some of the best examples of targeting the elements of the tumor microenvironment are inhibiting Hh, depleting high hyaluronan (HA) content, and targeting CD40.
Hh signaling is usually active during embryonic development and later regulates adult stem cells; it is notably reactivated early in pancreatic tumorigenesis.[68] Through depletion of stromal tissue, Hh inhibitors increase delivery of chemotherapy in mouse models.[69] However, multiple clinical trials have shown Hh inhibitors (vismodegib and saridegib) to yield inferior median PFS when combined with gemcitabine, compared with placebo or historical controls.[70,71] Perhaps this is because Hh inhibition in mouse models leads to more poorly differentiated and more aggressive tumors.[72] Moreover, depletion of tumor stromal myofibroblasts impairs the local immune response, perhaps offering another explanation for the poor clinical outcomes with Hh inhibitors.[73]
Another stromal targeted therapy that has recently shown promise uses pegylated recombinant human hyaluronidase (PEGPH20), which depletes HA in tumors; HA is a main component of the extracellular matrix and forms a barrier to chemotherapy.[74] An ongoing phase II study investigates whether the addition of PEGPH20 to standard gemcitabine and nab-paclitaxel improves outcomes in untreated metastatic pancreatic adenocarcinoma. Interim results of 146 patients are notable for improved median PFS in patients with HA-high tumors treated with PEGPH20 (9.2 vs 4.3 months; P = .05), and a trend towards a median OS benefit as well (12 vs 9 months; HR, 0.62 [95% CI, 0.26–1.46]).[75] Thromboembolic events occurred frequently in the PEGPH20 arm (28.4% vs 14.8%), but the trial was subsequently amended to exclude patients at high risk for thromboembolic events and to add enoxaparin prophylaxis, with no further thromboembolic events noted. A phase III trial in HA-high patients will start in early 2016.
CD40 agonists activate antigen-presenting cells, promoting an antitumor immune response. Monocytes infiltrate the tumor and degrade the stromal microenvironment, resulting in regression of the tumor.[76] A phase I study evaluated the fully human agonist CD40 monoclonal antibody CP-870,893 with full-dose gemcitabine in 22 patients with untreated advanced pancreatic cancer, demonstrating an ORR of 19% and stable disease in 50% of patients.[77] Cytokine release syndrome was a frequent adverse event, occurring in 91% of patients, but except for one grade 3 event, all instances were grade 1 or 2. Studies evaluating novel CD40 agonists in combination with checkpoint inhibitors are ongoing.
Finally, new approaches to targeting the tumor microenvironment are focusing on cancer stem cells. The stem cell factor inhibitor BBI608 blocks Stat3, which is critical for maintaining cancer stemness, while it spares hematopoietic stem cells in mouse xenograft models of pancreatic cancer.[78] A phase Ib trial of BBI608 with gemcitabine and nab-paclitaxel in untreated metastatic pancreatic adenocarcinoma (adjuvant therapy allowed) is currently ongoing, as are trials of BBI608 in other gastrointestinal (GI) malignancies. In addition, the novel agent necuparanib targets pathways critical for the tumor microenvironment, including P-selectin, CXCR4/stromal cell–derived factor 1, vascular endothelial growth factor/fibroblast growth factor 2, and heparanase. In a phase I trial of necuparanib combined with gemcitabine and nab-paclitaxel, O’Reilly et al demonstrated a 14.2-month OS and a disease control rate of 63%. There are plans to explore this promising combination in a randomized phase II trial.[79]
Interaction between the immune system and pancreatic cancer
Immunogenicity of pancreatic cancer. Immunotherapy is a very promising novel branch of cancer therapy that unfortunately has had little success in GI cancers, particularly in pancreatic ductal adenocarcinoma. There are several hypotheses as to why pancreatic adenocarcinoma has been difficult to treat using the immune system. First, as described previously, pancreatic ductal adenocarcinoma develops from a series of somatic gene mutations that generate altered proteins. Although one could hypothesize that these altered, mutated self-proteins may have antigenic potential, the reality may be that these antigens become tolerated by the immune system over time. Additionally, KRAS, the prototype oncogene that initiates a tumor’s chronic inflammatory state in pancreatic cancer, downregulates potential immune activity from any cells that attempt to lyse malignant cells.[80] Second, most GI cancers do not even induce an effector T-cell response that can be targeted immunologically. Tumor cells express immune mediators that block the activity of effector CD4+ and CD8+ T cells, and that dull the local immune response to tumor infiltration.[81] Finally, the tumor’s microenvironment tends to be an immune-suppressive setting.[82] In particular, the tumor’s stroma may provide a barrier to effector T-cell infiltration, and as a result, support invasion, migration, and tumor growth through a self-regulated inflammatory environment involving fibroblasts and secreted tumor-promoting factors.
Vaccines. Nevertheless, strategies to optimize immunotherapy against pancreatic ductal adenocarcinoma have been explored over the past decade. One such immunotherapeutic approach is tumor vaccination, for which clinical response has been limited. These vaccinations are generated based on overexpressed antigens in pancreatic ductal adenocarcinoma cells. Specifically, peptides, Listeria species, and dendritic cells have been the bases of the original vaccines tested; however, most of these strategies have not gone beyond phase I trials.[83] One of the most developed and promising vaccine therapies uses irradiated granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic pancreatic cancer cell lines (GVAX) to induce a T-cell response against pancreatic cancer cells; the vaccine is administered after low-dose cyclophosphamide in order to inhibit regulatory T cells.[84] A phase II study included 60 patients who were given the vaccine 8 to 10 weeks after surgical resection, followed by chemoradiation. The patients then continued the vaccine if they were disease-free after chemoradiation. Results of this study showed a slight improvement in median OS: 24.8 months, compared with 20.3 months in a historical control group of chemoradiation alone (95% CI, 21.2–31.6).[85] Another study confirmed the finding that mesothelin-specific CD8+ T-cell responses correlated with improved survival after whole-cell vaccination.[86] The majority of the literature on GVAX concludes that better outcomes occur when GVAX is combined with another vaccine or immune-modulating treatment. In fact, a phase II trial in patients with pretreated metastatic pancreatic adenocarcinoma showed a prolonged median OS (6.1 vs 3.9 months; P = .02) in patients who received low-dose cyclophosphamide → GVAX + CRS-207, a live-attenuated Listeria monocytogenes vaccine modified to express mesothelin (overexpressed on pancreatic cancer cells), compared with cyclophosphamide → GVAX alone.[87] An important neoadjuvant study in which GVAX was given with low-dose cyclophosphamide 2 weeks before surgical resection established that immunotherapy can convert a nonimmunogenic neoplasm into an immunogenic one by inducing infiltration of immune mediators into the tumor microenvironment, raising the hope that patients with vaccine-primed pancreatic ductal adenocarcinoma cells may be better candidates than vaccine-naive patients for immune checkpoint and other immunomodulatory therapies.[88] Currently, many vaccine strategies and combination therapies with vaccines are being tested in early-phase clinical trials.
Checkpoint blockade. Immune checkpoint inhibitors, such as the anti–cytotoxic T-lymphocyte–associated protein 4 (anti–CTLA-4) and anti–programmed death 1 (anti–PD-1) monoclonal antibodies, are FDA-approved for the treatment of melanoma and non–small-cell lung cancer. Programmed death ligand 1 (PD-L1) on the tumor cell’s surface modulates the immune system by dampening the local T-cell response and cytokine production during inflammation in order to avoid detection.[89] Previous phase II studies evaluating the use of anti–CTLA-4 or anti–PD-1/PD-L1 antibodies as sole treatments for pancreatic cancer were disappointing. Royal et al[90] showed only delayed regression (after new metastases formed) with ipilimumab in already established pancreatic tumors. No response came from treatment with anti–PD-L1 in pancreatic cancer patients.[91] However, the combination of checkpoint inhibitors with vaccine therapy holds promise for further improving outcomes in metastatic pancreatic adenocarcinoma, as there have been exciting results in other tumor types (eg, melanoma). GVAX (without cyclophosphamide) plus ipilimumab improved OS compared with ipilimumab alone in a phase Ib trial of 30 patients with pretreated advanced disease (5.7 vs 3.6 months; P = .072), although these results were not statistically significant.[92] STELLAR, a larger phase II trial of GVAX with cyclophosphamide and CRS-207, with or without nivolumab (an anti–PD-1 antibody), as second-line treatment in metastatic pancreatic adenocarcinoma is currently ongoing.[93]
Other methods of promoting tumor-infiltrating lymphocytes[94] may be used in combination with checkpoint inhibitors. One such method is the targeting of carcinoma-associated fibroblasts (CAFs) that express fibroblast activation protein. In theory, depleting chemokine (C-X-C motif) ligand 12 (CXCL12)-associated fibroblasts, a major part of stroma that promotes tumor growth, angiogenesis, and chemotherapeutic resistance, could allow immune control by breaking down the “stromal barrier,” creating favorable conditions for joint or adjuvant treatments. Many ongoing phase II studies are investigating immune checkpoint antibodies in combination with chemotherapy and vaccinations. Mouse models demonstrate that inhibition of CXCL12 receptor 4 results in depletion of CAFs and local accumulation of T cells, and synergistically unlocks the potential of anti–PD-L1 antibodies to stimulate T cells to conduct immune surveillance.[95] These findings must be further explored in a clinical trial. The use of chimeric antigen receptor (CAR)-modified T cells, re-engineered to recognize mesothelin, has also been studied in a phase I trial of 10 patients with chemorefractory metastatic pancreatic adenocarcinoma.[96] Treatment was well tolerated, without episodes of cytokine release syndrome, and two of the six treated patients had stable disease. Early studies of CAR T-cell therapy in metastatic pancreatic adenocarcinoma are ongoing.
Adoptive cell transfer. The idea of adoptive cell transfer involves removing the patient’s T cells, modulating them ex vivo, and then re-infusing the potent, more tumor-specific T cells. This method has been successful in bile duct cancer and is currently being evaluated in pancreatic ductal adenocarcinoma. The use of CAR-transduced T cells has had some clinical success in neuroblastoma patients, so a CAR against prostate stem cell antigen, a glycoprotein overexpressed in pancreatic ductal adenocarcinoma cells, was recently generated from human proteins. This study showed that human CAR induced a greater reactivity than mouse proteins, and that adoptive transfer of CAR T cells resulted in reduced tumor volume, confirming a potential to mediate a strong and systemic antitumor effect.[97]
Immunosuppressive factor inhibition. Also being investigated are inhibitors of indoleamine 2,3-dioxygenase (IDO; an immunosuppressive factor), which have been validated in mice.[98] IDO has been established as an important mediator of immune escape, and IDO inhibitors have been shown to trigger antitumor immunity and to act synergistically with various chemotherapeutic agents.[99] D1-MT, designed to target IDO, is entering phase I trials and may preferentially inhibit IDO2. This knowledge is useful in order to evaluate IDO2 as a marker not only for immune tolerance, but also for decreased susceptibility to D1-MT or similar compounds.[99] In addition, mTOR and protein kinase C have been identified as candidate pharmacodynamic markers for D1-MT response.[100] It has been demonstrated that IDO2 has a high frequency of functional single nucleotide polymorphisms in patients with pancreatic ductal adenocarcinoma,[101] and thus a simple genetic sequencing test could be used to determine upfront which patients might have a viable, active IDO2 that could be targeted for therapy.
Future Directions
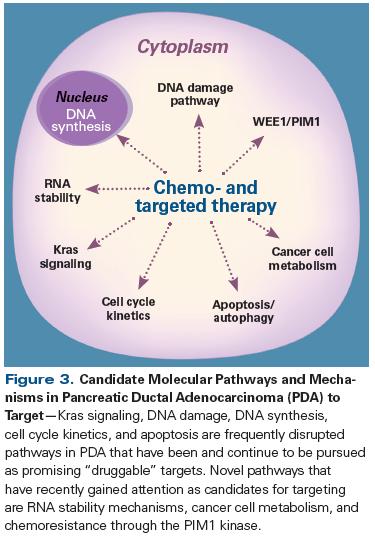
While there is clearly a long way to go, important strides have been made in the treatment of metastatic pancreatic adenocarcinoma. We now better understand the crucial interplay between tumor cells, stroma, and the immune system, as well as genetic and nongenetic drivers of tumorigenesis with potential drug targets (see Figure 3). The development of early detection and validated predictive biomarkers to aid in the selection of therapies is another priority that would benefit patients immediately. Also, more work needs to be done to elucidate the best sequence of immunotherapy agents, traditional chemotherapy, and surgical strategies. Despite slow progress historically, there is reason to be hopeful that, given the multitude of new experimental agents in the pipeline, along with our growing molecular understanding of pancreatic tumorigenesis, we are bound to see improvements in a disease with a heretofore dismal prognosis.
Financial Disclosure:Dr. Pishvaian receives speaking fees from and serves on the advisory board of Celgene; in addition, Dr. Pishvaian’s institution receives research funding from AbbVie, Celgene, Gilead, Merck, Novartis, Pfizer, and Threshold Pharmaceuticals. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. American Cancer Society: Cancer facts and figures 2015. http://www.cancer.org/research/cancerfactsfigures/index. Accessed October 12, 2015.
2. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-21.
3. Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843-52.
4. Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928-37.
5. Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23:1880-8.
6. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23:2964-70.
7. Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-53.
8. Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107:2005-9.
9. Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217.
10. Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790-5.
11. Rebours V, Boutron-Ruault MC, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111-9.
12. SEER Stat Fact Sheets: pancreas cancer. http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed October 12, 2015.
13. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-25.
14. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-703.
15. Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-13.
16. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-6.
17. Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-8.
18. Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-7.
19. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-16.
20. Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778-85.
21. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-22.
22. Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-10.
23. De Jesus-Acosta A, Oliver GR, Blackford A, et al. A multicenter analysis of GTX chemotherapy in patients with locally advanced and metastatic pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2012;69:415-24.
24. Fine RL, Fogelman DR, Schreibman SM, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol. 2008;61:167-75.
25. Conroy T, Paillot B, François E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer-a Groupe Tumeurs Digestives of the Fédération Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228-36.
26. James ES, Cong X, Yao X, et al. Final analysis of a phase II study of Yale-modified FOLFIRINOX (mFOLFIRINOX) in metastatic pancreatic cancer (MPC). J Clin Oncol. 2015;33(suppl 3):abstr 395.
27. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Pancreatic adenocarcinoma. Version 2.2015. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed October 12, 2015.
28. Ramanathan RK, Lee P, Leach JW, et al. Phase II study of induction therapy with gemcitabine and nab-paclitaxel followed by consolidation with mFOLFIRINOX in patients with metastatic pancreatic cancer. J Clin Oncol. 2013;31(suppl 4):abstr 233.
29. Portal A, Pernot S, Arbaud C, et al. Nab paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after failure of FOLFIRINOX: results of an AGEO multicenter prospective cohort. J Clin Oncol. 2015;33(suppl):abstr 4123.
30. Chen LT, Von Hoff DD, Li C-P, et al. Expanded analyses of napoli-1: phase 3 study of MM-398 (nal-IRI), with or without 5-fluorouracil and leucovorin, versus 5-fluorouracil and leucovorin, in metastatic pancreatic cancer (mPAC) previously treated with gemcitabine-based therapy. J Clin Oncol. 2015;33(suppl 3):abstr 234.
31. Borad MJ, Reddy SG, Bahary N, et al. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2015;33:1475-81.
32. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969-72.
33. Goggins M, Hruban RH, Kern SE. BRCA2 is inactivated late in the development of pancreatic intraepithelial neoplasia: evidence and implications. Am J Pathol. 2000;156:1767-71.
34. Hruban RH, Wilentz RE, Maitra A. Identification and analysis of precursors to invasive pancreatic cancer. Methods Mol Med. 2005;103:1-13.
35. Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39:91-100.
36. di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220-9.
37. Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744.
38. Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253-60.
39. Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397-402.
40. van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res. 2005;11:7508-15.
41. van der Heijden MS, Brody JR, Gallmeier E, et al. Functional defects in the Fanconi anemia pathway in pancreatic cancer cells. Am J Pathol. 2004;165:651-7.
42. van der Heijden MS, Yeo CJ, Hruban RH, Kern SE. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003;63:2585-8.
43. Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501.
44. Pishvaian MJ, Wang H, Zhuang T, et al. A phase I/II study of ABT-888 in combination with 5-fluorouracil (5-FU) and oxaliplatin (Ox) in patients with metastatic pancreatic cancer (MPC). J Clin Oncol. 2012;30(suppl 4):abstr 147.
45. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-50.
46. Kindler HL, Locker GY, Mann H, et al. POLO: A randomized phase III trial of olaparib tablets in patients with metastatic pancreatic cancer (mPC) and a germline BRCA1/2 mutation (gBRCAm) who have not progressed following first-line chemotherapy. J Clin Oncol. 2015;33(suppl):abstr TPS4149.
47. Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126-30.
48. Shain AH, Giacomini CP, Matsukuma K, et al. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc Natl Acad Sci USA. 2012;109:E252-E259.
49. Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES. The meaning of p16(ink4a) expression in tumors: functional significance, clinical associations and future developments. Cell Cycle. 2011;10:2497-503.
50. Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget. 2014;5:6512-25.
51. Imrie CW. Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology. 2015;15:327-30.
52. Hurwitz H, Uppal N, Wagner SA, et al. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC). J Clin Oncol. 2014;32(suppl 5s):abstr 4000.
53. Hurwitz H, Garrett WM, Clark J, et al. JANUS 1: a phase 3 placebo-controlled study of ruxolitinib plus capecitabine in patients with advanced or metastatic pancreatic cancer (mPC) after failure or intolerance of first-line chemotherapy. J Clin Oncol. 2015;33(suppl):abstr TPS4147.
54. O’Reilly EM, Walker C, Clark J, et al. JANUS 2: a phase III study of survival, tumor response, and symptom response with ruxolitinib plus capecitabine or placebo plus capecitabine in patients with advanced or metastatic pancreatic cancer (mPC) who failed or were intolerant to first-line chemotherapy. J Clin Oncol. 2015;33(suppl):abstr TPS4146.
55. Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901-8.
56. Sicard F, Gayral M, Lulka H, et al. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986-94.
57. Lal S, Burkhart RA, Beeharry N, et al. HuR posttranscriptionally regulates WEE1: implications for the DNA damage response in pancreatic cancer cells. Cancer Res. 2014;74:1128-40.
58. Richards NG, Rittenhouse DW, Freydin B, et al. HuR status is a powerful marker for prognosis and response to gemcitabine-based chemotherapy for resected pancreatic ductal adenocarcinoma patients. Ann Surg. 2010;252:499-505; discussion 05-6.
59. Williams TK, Costantino CL, Bildzukewicz NA, et al. pp32 (ANP32A) expression inhibits pancreatic cancer cell growth and induces gemcitabine resistance by disrupting HuR binding to mRNAs. PLoS One. 2010;5:e15455.
60. Rajeshkumar NV, De Oliveira E, Ottenhof N, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799-806.
61. Burkhart RA, Pineda DM, Chand SN, et al. HuR is a post-transcriptional regulator of core metabolic enzymes in pancreatic cancer. RNA Biol. 2013;10:1312-23.
62. Blanco FF, Jimbo M, Walfkuhle J, et al. The mRNA-binding protein HuR promotes hypoxia-induced chemoresistance through posttranscriptional regulation of the proto-oncogene PIM1 in pancreatic cancer cells. Oncogene. 2015 Sep 21. [Epub ahead of print]
63. Costantino CL, Witkiewicz AK, Kuwano Y, et al. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009;69:4567-72.
64. Pineda DM, Rittenhouse DW, Valley CC, et al. HuR’s post-transcriptional regulation of Death Receptor 5 in pancreatic cancer cells. Cancer Biol Ther. 2012;13:946-55.
65. Jimbo M, Blanco FF, Huang YH, et al. Targeting the mRNA-binding protein HuR impairs malignant characteristics of pancreatic ductal adenocarcinoma cells. Oncotarget. 2015;6:27312-31.
66. Meisner NC, Hintersteiner M, Mueller K, et al. Identification and mechanistic characterization of low-molecular-weight inhibitors for HuR. Nat Chem Biol. 2007;3:508-15.
67. Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-76.
68. Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851-6.
69. Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-61.
70. Kim EJ, Sahai V, Abel EV, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937-45.
71. Madden J. Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer [press release]. Jan 27 2012. http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle&ID=1653550. Accessed October 12, 2015.
72. Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-47.
73. Ãzdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-34.
74. Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-29.
75. Hingorani SR, Harris WP, Hendifar AE, et al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: interim results of a randomized phase II study. J Clin Oncol. 2015;33(suppl):abstr 4006.
76. Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-6.
77. Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286-95.
78. Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA. 2015;112:1839-44.
79. O’Reilly EM, Mahalingam D, Roach JM, et al. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of necuparanib combined with nab-paclitaxel and gemcitabine in patients with metastatic pancreatic cancer: phase 1 results. J Clin Oncol. 2015;33(suppl):abstr 4114.
80. Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J Gastroenterol. 2014;20:11160-81.
81. Wang J, Reiss KA, Khatri R, et al. Immune therapy in GI malignancies: a review. J Clin Oncol. 2015;33:1745-53.
82. Di Caro G, Castino GF, Bergomas F, et al. Immune-based therapies in pancreatic and colorectal cancers and biomarkers of responsiveness. Expert Rev Anticancer Ther. 2014;14:1219-28.
83. Salman B, Zhou D, Jaffee EM, et al. Vaccine therapy for pancreatic cancer. Oncoimmunology. 2013;2:e26662.
84. Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455-63.
85. Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-35.
86. Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297-306.
87. Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325-33.
88. Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616-31.
89. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800.
90. Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-33.
91. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-65.
92. Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-9.
93. Le DT, Crocenzi TS, Uram JN, et al. Randomized phase II study of the safety, efficacy, and immune response of GVAX pancreas vaccine (with cyclophosphamide) and CRS-207 with or without nivolumab in patients with previously treated metastatic pancreatic adenocarcinoma (STELLAR). J Clin Oncol. 2015;33(suppl):abstr TPS4148.
94. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62-8.
95. Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212-7.
96. Beatty GL, O’Hara MH, Nelson AM, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemorefractory metastatic pancreatic cancer. J Clin Oncol. 2015;33(suppl):abstr 3007.
97. Abate-Daga D, Rosenberg SA, Morgan RA. Pancreatic cancer: hurdles in the engineering of CAR-based immunotherapies. Oncoimmunology. 2014;3:e29194.
98. Manuel ER, Chen J, D’Apuzzo M, et al. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immunol Res. 2015 Jul 1. [Epub ahead of print]
99. Metz R, Duhadaway JB, Kamasani U, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082-7.
100. Metz R, Rust S, Duhadaway JB, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460-8.
101. Witkiewicz AK, Costantino CL, Metz R, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg. 2009;208:781-7; discussion 87-9.