Several prospective randomized clinical trials conducted internationally have proven the safety and survival equivalence of breast-conserving surgery compared with mastectomy. Adjuvant radiation is routinely recommended following lumpectomy surgery to minimize the risk of local recurrence. Comprehensive breast imaging (including bilateral mammography with diagnostic views and ultrasound evaluation), in addition to clinical examination, is essential to rule out potential contralateral pathology and to optimally characterize the extent of disease. These studies are considered standard in the assessment of patient eligibility for lumpectomy. MRI of the breast remains controversial as an adjunct to determine candidacy for breast conservation, since MRI findings increase mastectomy rates without evidence of improved local control; prospective randomized clinical trials are underway to define the role of MRI in newly diagnosed breast cancer. Recently, the multidisciplinary oncology community has adopted a consensus guideline defining “no ink on tumor” as an acceptable microscopic margin at lumpectomy; however, post-lumpectomy imaging may be necessary to confirm complete removal of all cancer-associated microcalcifications, with clinical judgment exercised regarding re-excision for close margins. Contralateral prophylactic mastectomy is becoming increasingly common in the United States, and patients considering this option must be counseled about its lack of a survival benefit, its higher complication rate, and the fact that it is risk-reducing but not risk-eliminating.
General Concepts in Primary Management of Invasive Breast Cancer
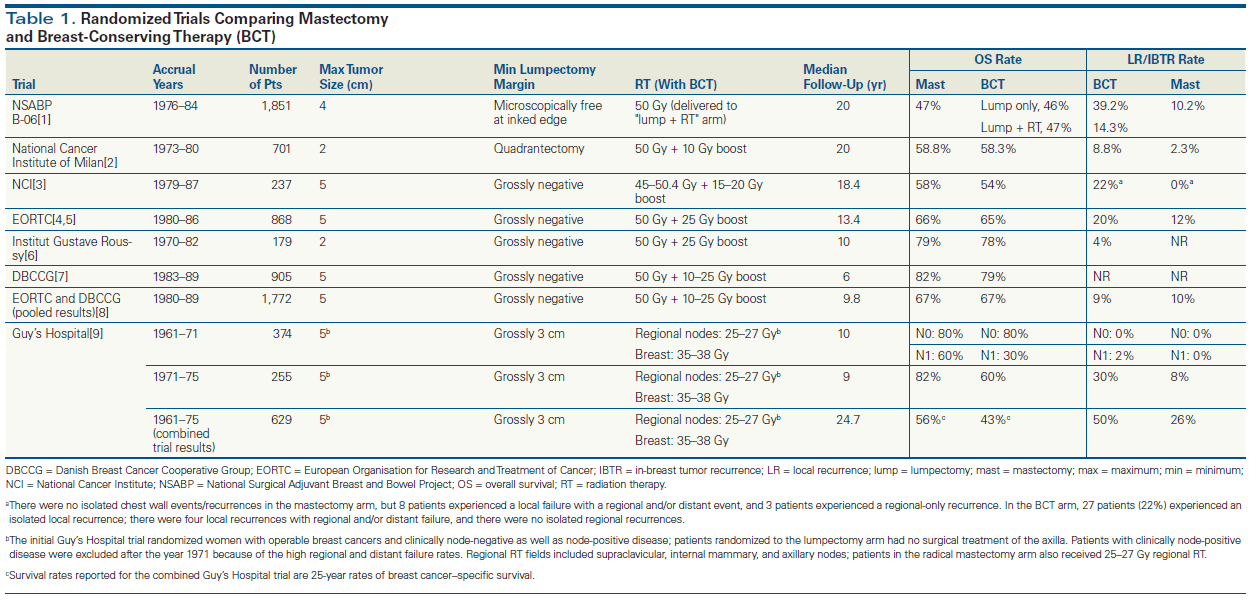
Multiple international randomized clinical trials conducted between 1961 and 1989 confirmed the equivalence of overall survival outcomes between breast-conserving surgery and mastectomy for operable breast cancer (Table 1).[1-9] As shown by the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 trial, adjuvant radiation therapy (RT) does not have a significant impact on overall survival, but it reduces the risk of in-breast recurrence substantially.[1] Lumpectomy followed by contemporary whole-breast RT (WBRT) is associated with an average annual risk of subsequent in-breast cancer events ranging from 0.3% to 1%, and at 5 years local recurrences are therefore observed in 2% to 5% of cases. During the first 5 to 10 years of follow-up, most of these in-breast events are true local recurrences; the ongoing cumulatively increasing risks represent the progressively rising likelihood of developing a new primary breast cancer. Patients with hereditary susceptibility to breast cancer as a consequence of harboring a BRCA1 or BRCA2 mutation may have a risk of new primary breast cancer as high as 2% to 5% per year.[10,11] The chemoprevention and local control benefits conferred by the use of adjuvant endocrine therapies reduce the risk of new primary breast cancer by 50% for patients treated with tamoxifen, and by 70% for patients who receive treatment with aromatase inhibitors.[12] Notably, however, endocrine therapies only reduce the risks associated with hormone receptor (HR)-positive breast cancer, and carriers of BRCA1 mutations are at increased risk for developing triple-negative breast cancers (TNBCs; tumors that are negative for the estrogen receptor, the progesterone receptor, and human epidermal growth factor receptor 2), for which endocrine therapies are ineffective.
As shown in Table 1, several of the prospective randomized trials demonstrated rates of ipsilateral breast cancer events that were twofold to fourfold higher following lumpectomy and radiation, compared with the rates of chest wall failure following mastectomy. In general, local recurrence after mastectomy is perceived as being an indicator of biologically aggressive disease, and a harbinger of distant organ failure. In contemporary breast cancer management, patients who present with clinical features suggesting increased risk of chest wall recurrence are recommended to receive postmastectomy RT. Chest wall failures at 10 years are observed in more than 20% to 30% of patients with at least four metastatic axillary nodes, node-positive T3 disease, inflammatory breast cancer, and/or residual axillary metastases following neoadjuvant chemotherapy. Postmastectomy RT can reduce these rates by more than 50% and therefore is routinely recommended in these scenarios. Benefits of postmastectomy RT are less well defined in T1/T2 breast cancer associated with one to three positive axillary nodes, T3/node-negative breast cancer, and in patients presenting with axillary node–positive disease that is downstaged to node negativity by neoadjuvant chemotherapy.[13]
Along with improvements in surgical management of the breast in patients with cancer, surgical management of the axilla has evolved significantly, with less invasive approaches available in select patient populations. Lymphatic mapping with sentinel lymph node (SLN) biopsy has emerged as the preferred strategy for assessing nodal status.[14] Plastic surgeons have developed a broad array of reconstruction options for mastectomy patients, as well as techniques to restore symmetry in selected patients who have undergone lumpectomy with or without RT. Multidisciplinary breast oncology in the 21st century combines the expertise of breast imaging specialists; pathologists; and medical, surgical, and radiation oncologists to offer optimized systemic therapy and surgical management with the least disfigurement.
Potential Advantages of Breast-Conserving Surgery vs Mastectomy
Multiple studies have documented rising rates of mastectomy in the United States among breast cancer patients who are candidates for lumpectomy; there has also been an increase in the number of patients undergoing contralateral prophylactic mastectomy (CPM), despite the lack of definitive evidence that this procedure confers a survival advantage.[15] Patient choice must be respected as an essential element of quality of life; however, given these changing patterns of care, surgeons must carefully communicate to the individual patient all of the appropriatebreast cancer treatment options and confirm that they are understood.[16,17] Indeed, breast-conserving surgery generates definitive staging information without “burning any bridges” and has other advantages compared with a potentially premature decision to pursue mastectomy.
For example, lumpectomy in conjunction with axillary surgery as the initial treatment plan provides the patient and multidisciplinary team with definitive histopathologic information regarding the primary tumor and lymph nodes. The status of the axillary lymph nodes in particular can have a significant downstream impact on other surgical treatment decisions. Patients who undergo lumpectomy and are shown to have metastatic disease in one or two SLNs can usually be spared from undergoing a completion axillary lymph node dissection (ALND). Results from the American College of Surgeons Oncology Group Z0011 trial demonstrated that completion ALND provides no advantage in clinical outcomes to such patients,[18] with observed equivalence of locoregional control likely related to adjuvant irradiation of the lower (undissected) nodal basin of the breast. In contrast, patients for whom mastectomy is planned usually do require completion ALND if SLN metastases are present, since knowing the fully quantified extent of nodal metastatic disease will enable clinicians to determine whether RT will be necessary following mastectomy.[14] Furthermore, node-positive mastectomy patients are often discouraged from pursuing immediate breast reconstruction because some plastic surgeons are unwilling to accept the risk of potential irreversible damage to the reconstructed breast caused by postmastectomy radiation.
Therefore, performing an initial lumpectomy and SLN biopsy preserves more options, namely:
• Patients found to be node-negative can pursue mastectomy (if strongly desired) and immediate reconstruction free of concerns regarding postmastectomy RT.
• As described previously, patients with limited nodal metastases can avoid completion ALND and need not face the prospect of mastectomy without immediate reconstruction.
• Mastectomy with immediate reconstruction remains an option as elective prophylactic surgery following completion of the acute multimodality cancer treatment protocol.
The multidisciplinary management team must clarify several aspects of breast cancer care that are commonly misunderstood by newly diagnosed patients. Often, patients are under the mistaken impression that mastectomy is a more “aggressive” approach to treating their cancer, simply because it represents more extensive surgery. Similarly, patients often assume that mastectomy provides a guarantee that they will never have to deal with breast cancer again, or that undergoing this surgery will help them to avoid needing chemotherapy. These misperceptions will not necessarily be verbalized, so it is incumbent upon the clinician to clearly communicate the following facts to the patient:
• Survival is equivalent for breast-conserving surgery and mastectomy.
• Mastectomy does not eliminate the risk of local recurrence or new primary cancer.
• Chemotherapy recommendations are independent of the decision to pursue mastectomy vs lumpectomy.
• RT may still be indicated following mastectomy.
Preoperative Workup to Determine Eligibility for Lumpectomy
Progress toward successful breast-conserving surgery begins with the diagnostic biopsy. Every attempt should be made to establish the cancer diagnosis via percutaneous core needle biopsy. Patients whose cancer diagnosis is confirmed by needle biopsy can then efficiently proceed to planning for definitive breast surgery performed concomitantly with the appropriate axillary lymph node surgery. Surgical breast biopsies represent the most definitive diagnostic sampling approach (and are always indicated when needle biopsies are either not feasible or yield a discordant result), but they are less efficient than needle biopsies. For example, surgical breast biopsies revealing invasive disease usually have inadequate margins because the surgeon has not planned for a cancer-directed resection, and patients will also require additional surgery for axillary staging.[19]
When needle biopsies are feasible, it is preferable to obtain multiple core specimens rather than obtaining samples using fine needle aspiration/cytology. Core needle biopsies provide a greater amount of tissue (resulting in a higher diagnostic yield); can be used to distinguish invasive from in situ disease; and are better suited for immunohistochemistry, to evaluate biomarker expression on the invasive component. Easily palpable tumors can be examined using freehand core needle biopsies; even in such cases, however, image-guided biopsies are advantageous because they have better diagnostic accuracy and can be performed with deployment of a radio-opaque clip left in the cancer bed. These clips facilitate subsequent surgical management in patients who are candidates for neoadjuvant chemotherapy.
Conventionally accepted guidelines suggest that lumpectomy can be safely offered to patients with T1 and T2 breast cancers; however, it is worth noting that patients were excluded from participating in the NSABP B-06 trial if they had primary tumors larger than 4 cm.[1] In recognition of the preponderance of data demonstrating that poor local control of tumor progression is an indicator of risk for distant relapse but is not a precursor of metastatic spread, it is now widely accepted that patient eligibility for lumpectomy is determined more by the anticipated cosmetic result, as well as the ability to achieve margin control and deliver adjuvant radiation, than by an arbitrarily selected threshold for the size of the primary tumor.[20] Tumor location and other anatomic features specific to the individual patient may influence the relative acceptability of lumpectomy.
Body habitus and the tumor-to-breast volume ratio often have an impact on the choice of surgical approach. If the patient has relatively small breasts, conservative therapy for small tumors may yield an unsatisfactory cosmetic result, given the breast volume loss secondary to a margin-negative lumpectomy and tissue retraction due to radiation-related fibrosis. At the other end of the spectrum, a T3 cancer may be amenable to breast conservation in a large and/or pendulous breast. In this setting, breast conservation may actually be preferred because of the quality-of-life challenges associated with unilateral mastectomy in a large-breasted woman. The resulting chest wall imbalance and dependence on a heavy prosthesis to match the remaining breast may enhance the patient’s level of interest in breast conservation. Severe morbid obesity, however, may preclude eligibility for lumpectomy because treatment systems in most RT facilities will have a weight limit. If mastectomy is necessary, then options for breast reconstruction or contralateral reduction mammoplasty should be explored. Selected patients may opt for bilateral mastectomy if they find that a symmetrically flat chest wall simplifies the daily activity of getting dressed.
Breast imaging is essential for determining patient eligibility for lumpectomy. Clinicians should scrutinize the bilateral mammogram, carefully evaluating it for the size of the primary tumor, evidence of multicentric disease, the extent of malignant-appearing microcalcifications, and possible contralateral synchronous breast cancer. Patients with diffuse, malignant-appearing calcifications and/or calcifications that cannot be encompassed by a lumpectomy specimen should be referred for mastectomy. When benign or indeterminate microcalcifications are identified on the preoperative mammogram, decisions must be made regarding whether to perform image-guided needle biopsy of the additional foci, and clinicians must determine whether these additional areas would be amenable to mammographic surveillance if left unresected. Other suspicious foci should be biopsied if demonstration of cancer would affect eligibility for lumpectomy or if the patient is a candidate for preoperative chemotherapy. In the latter case, biomarker status of other tumor sites must be documented prior to delivery of systemic therapy, and insertion of image-guided clips may influence the extent of breast resection performed after neoadjuvant treatment. If lumpectomy is attempted in the patient with cancer-associated microcalcifications, then it is necessary to obtain a post-lumpectomy mammogram to confirm the adequacy of the resection. The presence of residual microcalcifications is an indication for additional breast surgery (either re-excision lumpectomy or mastectomy) regardless of whether margin control was achieved with the prior lumpectomy.
Targeted breast ultrasound is useful for precise estimation of the size of the primary invasive tumor. Mammography results are sometimes misleading because of overestimation of the extent of microcalcifications (which might actually represent coexisting noninvasive disease) or of the span of spiculations representing retraction of adjacent normal breast parenchyma.
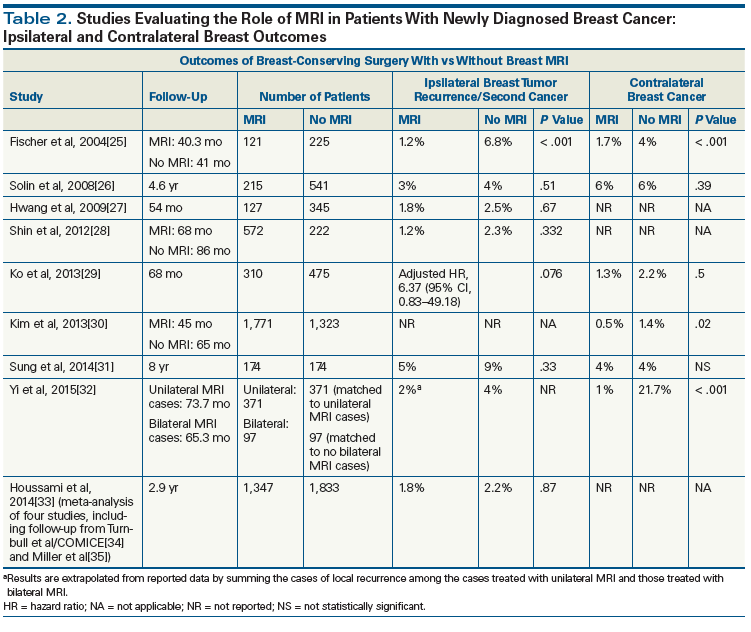
Ongoing controversy exists regarding the potential role of breast MRI in the setting of a newly diagnosed breast cancer. MRI has confirmed value as a screening/early detection strategy in women with hereditary susceptibility for breast cancer[21] and in identifying candidates for breast-conserving surgery among patients presenting with axillary metastases but an occult primary breast tumor.[22] While performing breast MRI in patients with newly diagnosed breast cancer presumably could improve the process of selecting candidates for lumpectomy, this imaging modality might also have the unwanted effect of identifying foci of disease beyond the lumpectomy bed that would have been eradicated by adjuvant radiation, possibly leading to unnecessary aggressive management. Indeed, several studies have confirmed that breast MRI results in higher rates of mastectomy when performed in patients with newly diagnosed breast cancer.[22-24] As shown in Tables 2 and 3, many retrospective comparisons of patients who did or did not undergo MRI procedures prior to their breast conservation surgery fail to demonstrate reduced rates of either local recurrence or re-excision as a result of MRI examinations.[24-39] Most of these studies also reveal similar rates of contralateral breast cancers. The Alliance clinical trial cooperative group’s study A011104, which will randomize use of breast MRI in women with HR-negative breast cancer, is expected to provide high-level evidence regarding the potential role of breast MRI in treatment planning.
Absolute and Relative Contraindications to Breast-Conserving Surgery
Among patients who, clinically and radiographically, appear to be candidates for lumpectomy, most contraindications to breast-conserving surgery are related either to the inability to obtain margin control by lumpectomy and/or re-excision(s) or the inability to administer RT to the patient. Examples of contraindications to RT include prior therapeutic radiation exposure, pregnancy, and selected connective tissue disorders. The final subsection will explore clinicopathologic features that should not be considered contraindications to breast conservation.
Lumpectomy margin control
The original prospective randomized clinical trials establishing the safety of breast-conserving surgery varied substantially in their management of lumpectomy margins (with margin width being the distance from the cancer cells to the surfaces of the excision specimen). Patients enrolled in the Milan-based trial by Veronesi et al,[2] supported by the Italian Association for Cancer Research, presumably had the most widely negative margins, because participants had tumor sizes no larger than 2 cm, and all underwent relatively aggressive quadrantectomy resections. At the other end of the spectrum, the study by investigators from the National Cancer Institute in the United States[3] involved gross “tumorectomy” resections, with no predefined mandate regarding margins. In between these extremes, the NSABP B-06 trial[1] required patients to have margin-negative lumpectomies, defined as the absence of cancer at the inked margin surfaces.
KEY POINTS
- Breast-conserving surgery (lumpectomy and radiation therapy) is equivalent to mastectomy with regard to overall survival.
- Standard mammographic (bilateral) and ultrasound imaging is essential for optimal selection of lumpectomy candidates and to rule out contralateral disease; the role of breast MRI is controversial and is associated with increased mastectomy rates.
- Contralateral prophylactic mastectomy has not been definitively shown to have any survival benefits, but selected patients may opt for this surgery as a risk-reducing (but not risk-eliminating) procedure.
In the decades following completion of the trials of breast-conserving surgery, there were no universally accepted definitions for optimal negative margin thickness, and individual facilities adhered to their own standards. This approach generated substantial inconsistencies between hospitals and oncology programs, with many facilities adhering to the classic NSABP definition of tumor-free inked margins, but with others subscribing to the theory that a more widely negative margin will improve local control, and mandating minimum margin thicknesses of 1 mm, 2 mm, or even 5 mm. Recently, however, the major academic and professional societies representing surgical oncology (Society of Surgical Oncology), radiation oncology (American Society for Radiation Oncology), and medical oncology (American Society of Clinical Oncology) collaborated to establish consensus regarding an oncologically acceptable lumpectomy margin, which was defined as the “no tumor on ink” standard utilized by the NSABP investigators.[40,41] The resulting consensus statement was based upon findings from a meta-analysis evaluating local control as a function of margin status in 33 studies involving 1,506 local recurrences in 28,162 cases.[42] While a positive margin was clearly a risk factor for local recurrence, there was no significant improvement in local control associated with obtaining more widely negative margins beyond clearance of the inked surfaces. It is important to bear in mind, however, that this multidisciplinary consensus statement represents a guideline, and exercising clinical judgment remains important. Selected patients with multiple close margins, especially in the setting of an extensive intraductal component (ductal carcinoma in situ [DCIS]), may be reasonably considered candidates for re-excision because of concerns that these ductal patterns may indicate an in-breast disease burden that will be less well controlled by RT.
Prior RT
Patients with newly diagnosed breast cancer and a history of RT to the chest wall are typically referred for mastectomy, because of the toxicity related to exceeding radiation threshold doses, and because of reduced local control of tumors managed with lumpectomy alone.
Patients with Hodgkin lymphoma have often received mantle radiation to the chest wall, which is a confirmed risk factor for breast cancer if the RT is delivered during adolescence or early adulthood. The risk of breast cancer in this setting is estimated to be 35% by the age of 50 years.[43]
Patients treated for prior breast cancer with lumpectomy and standard WBRT are also at risk for subsequent in-breast events, in the form of either a true local recurrence or a new primary cancer. Either pattern of subsequent ipsilateral disease generally results in referral for mastectomy. The Radiation Therapy Oncology Group (RTOG) 1014 trial is a phase II study now underway that aims to analyze the effectiveness of re-irradiation to control local recurrences in patients who have undergone repeat breast-conserving surgery (ClinicalTrials.gov identifier: NCT01082211). Currently the safety of repeat breast-conserving surgery in lumpectomy patients treated with prior partial breast RT is uncertain.
Pregnancy
The occurrence of breast cancer in pregnant women carries several unique challenges.[44,45] Management options vary by trimester and fetal development, but radiation is contraindicated throughout pregnancy. Ultrasound evaluation of the breast/axilla is harmless during pregnancy, and mammographic imaging can be performed with pelvic/fetal shielding. Chemotherapy can be safely delivered during the second and third trimester, and patients with definitive indications for receiving chemotherapy can defer locoregional treatments until after elective induced labor and delivery (which is timed to avoid spontaneous labor during the chemotherapy nadir). During the second and third trimester of pregnancy, women with breast cancer can be managed with primary lumpectomy and axillary surgery followed by adjuvant chemotherapy, or with primary/neoadjuvant chemotherapy followed by postpartum surgery and breast irradiation.
Breast cancer diagnosed during the first trimester, and breast cancer without definitive indications for chemotherapy (during any trimester) can only be managed with surgery. In such cases, mastectomy is the preferred approach, unless the diagnosis is made late in the third trimester, in which case lumpectomy and axillary surgery can be performed with RT delivered postpartum.
The performance of SLN biopsy requires extra caution in pregnant patients with breast cancer, because of uncertainty regarding effects of the commercially available blue dyes used in this procedure on the fetus. Phantom studies indicate that radioisotope-guided SLN mapping is associated with minimal pelvic exposure to radiation. Technetium-labeled sulfur colloid is generally considered to be a reasonable option for lymphatic mapping and SLN biopsy in staging pregnant breast cancer patients.
Connective tissue disorders
Scleroderma and Sjögren syndrome are connective tissue/collagen vascular disorders associated with cutaneous fragility and contraindication to radiation because of the resulting excessive toxicity of treatment.[46] Breast cancer patients with a pre-existing history of these disorders are usually referred for mastectomy. The presence of systemic lupus erythematosus or rheumatoid arthritis is believed by some to confer additional risk of radiation toxicity in patients with breast cancer; however, evidentiary data have been sparse, and so breast cancer management is individualized after assessing for any history of cutaneous manifestations.
Barriers to healthcare access
Standard external-beam WBRT requires a commitment to receiving daily fractionated radiation doses over a period of several weeks. Geographic factors may therefore create inherent barriers to safe delivery of breast-conserving surgery. Patients residing in remote areas may not have convenient access to a radiation facility. Socioeconomically disadvantaged patients may have transportation problems that preclude their ability to comply with RT treatment schedules.
Factors that are not contraindications to breast-conserving surgery
Several clinicopathologic features that may influence a patient’s choice between mastectomy and lumpectomy are actually not contraindications to breast-conserving surgery. Examples include younger age at diagnosis, hereditary susceptibility, histology, central/subareolar tumor location, and phenotype.
Younger age at breast cancer diagnosis. Bilateral mastectomy is often preferred among relatively younger patients with breast cancer, notably those with known hereditary susceptibility to the disease, such as BRCA1/BRCA2 mutation carriers. Because these groups are at increased risk for developing metachronous primary breast cancers, bilateral mastectomy serves a risk-reducing purpose.[11] However, patients contemplating bilateral mastectomy must understand that survival rates tend to be driven by the metastatic potential of the first cancer, and that mastectomy does not eliminate the risk of either chest wall/local recurrence (which is a function of tumor biology) or the development of a new primary (because microscopic foci of breast tissue can remain in skin flaps or in the axilla).
Lobular histology. Because of the insidious clinical manifestations of tumors with a lobular histology, it is more difficult to perform successful breast-conserving surgery in these patients.[47-49] Invasive lobular breast cancers are often associated with vague densities on clinical examination as well as on imaging, making margin control more challenging. As long as negative margins can be obtained, however, local control of invasive lobular cancers is comparable to that in ductal cancers. The presence of lobular carcinoma in situ (LCIS) coexisting with an invasive cancer or DCIS does not affect rates of local recurrence either; LCIS at lumpectomy margins has no clinical significance and does not warrant a re-excision. Pleomorphic LCIS, on the other hand, behaves more like DCIS and does require margin clearance.
Central/subareolar tumor location. The presence of centrally located tumors involving the subareolar tissue and/or nipple has previously been considered a relative contraindication to breast-conserving therapy because of the need for nipple removal. However, if disease is confined to a central unifocal area, without diffuse microcalcifications, and if margin negativity can be achieved, then performing a central segmentectomy is a reasonable approach. Elective nipple-areolar reconstruction can proceed following completion of breast RT.
Breast tumor phenotype. Advances in gene expression profiling have improved our insights into the heterogeneity of breast tumor subtypes. Most immunohistochemically defined TNBCs belong to the inherently aggressive basal and claudin-low breast cancer subtypes. The virulence of TNBC has prompted questions regarding whether they would be better controlled by mastectomy; however, studies have demonstrated that TNBC is a more challenging disease in terms of both locoregional and distant control, regardless of whether mastectomy or breast-conserving surgery is pursued. Breast tumor phenotype therefore does not determine eligibility for lumpectomy.[50]
Bilateral/Contralateral Prophylactic Mastectomy
Newly diagnosed breast cancer patients should be routinely assessed for lumpectomy eligibility as a first step in disease management. Patients who are not candidates for lumpectomy and/or breast radiation then need to be counseled regarding mastectomy and reconstruction options. The option of nipple preservation has motivated additional interest in the option of mastectomy with immediate reconstruction.
Optimal candidates for nipple-sparing mastectomy include breast cancer patients with lower risk of harboring microscopic disease in the central skin, such as patients with small, unifocal tumors located at least 2 cm from the nipple-areolar complex. No prospective randomized controlled trials have directly compared nipple-sparing with nipple-sacrificing mastectomy, and systematic reviews have not yet definitively demonstrated the safety of nipple-areolar preservation in the setting of cancer.[51,52]
Risk-reducing CPM should only be considered for patients who are unwilling or unable to pursue breast-conserving surgery for the biopsy-proven cancerous breast. Increasing rates of bilateral mastectomy among women with unilateral disease may reflect widespread overestimation of the benefits of CPM. Young age, lobular histology, familial cancer, white American background, affluence, and preoperative MRI have all been associated with higher rates of CPM.[53,54] Interestingly, one study from Switzerland suggested that CPM is more popular in the United States than in Europe.[55]
CPM does have a few potential benefits. Some patients prefer the symmetry of bilateral reconstruction. Further, the transabdominal myocutaneous flap reconstruction can only be utilized once, but it can be used for bilateral mastectomy reconstruction if the patient has adequate abdominal donor tissue (in terms of the amount of well-vascularized skin and fat) and if both breasts are reconstructed synchronously.[56] As noted previously, selected large-breasted women requiring mastectomy may request CPM to avoid the imbalance of unilateral surgery if they are not candidates for reconstruction or contralateral reduction mammoplasty. Some patients remain committed to CPM to maximally reduce the likelihood of having to repeat the breast cancer diagnosis and treatment experience.
Disadvantages of CPM include the more prolonged duration of surgery and higher associated complication rates. Moreover, patients must understand that CPM does not confer 100% protection against a new primary breast cancer; in any event, reducing the risk of a second primary breast cancer does not necessarily translate into a survival advantage. CPM does not affect risk of local recurrence from the known unilateral breast cancer.
Historically, most reported series have demonstrated that the survival rates from metachronous bilateral breast cancer are generally driven by the stage of the first cancer diagnosed and the treatment regimen employed.[57-65] This finding is biologically plausible because the first cancer will have a lead-time advantage in establishing micrometastatic disease, and it might be surmised that the initially presenting cancer is associated with the faster-growing pathology. Further, patients who have been treated for breast cancer will probably be observed more closely; therefore, a second tumor is more likely to be detected at an early stage, effectively controlled, and treatable with breast-conserving surgery.
Selected retrospective studies have revealed a possible survival advantage associated with CPM.[66-71] This observation may reflect improvements in systemic therapy for breast cancer, yielding a larger pool of patients whose initial micrometastatic burden is “cured,” thereby leaving them at increased mortality risk from a second breast cancer. The chemoprevention benefits of adjuvant therapies, with reduced incidence of metachronous disease, must be weighed against this theory. Further, several recent studies have revealed mostly comparable outcomes for patients undergoing CPM compared with those undergoing ipsilateral breast cancer surgery only,[54,72,73] and still other investigators have suggested the mitigating effect of selection bias, with CPM patients having improved breast cancer survival, as well as non–breast cancer survival.[74]
Summary and Conclusions
Breast-conserving surgery and mastectomy are equivalent treatments for invasive breast cancer patients in terms of overall survival. For the majority of patients, successful breast conservation requires a margin-negative lumpectomy and access to WBRT. Preoperative imaging with bilateral mammography to assess eligibility for lumpectomy (often accompanied by targeted ultrasound) is essential; preoperative breast MRI has uncertain value and increases the likelihood of surgeons performing mastectomy on their patients with breast cancer. CPM yields no definitive survival advantage and should only be considered in highly motivated patients who are unable or unwilling to undergo breast-conserving surgery for the index cancer.
Financial Disclosure:The author has no significant interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-41.
2. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-32.
3. Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98:697-702.
4. van Dongen JA, Bartelink H, Fentiman IS, et al. Factors influencing local relapse and survival and results of salvage treatment after breast-conserving therapy in operable breast cancer: EORTC trial 10801, breast conservation compared with mastectomy in TNM stage I and II breast cancer. Eur J Cancer. 1992;28A:801-5.
5. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143-50.
6. Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14:177-84.
7. Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;11:19-25.
8. Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688-97.
9. Fentiman IS. Long-term follow-up of the first breast conservation trial: Guy’s wide excision study. Breast. 2000;9:5-8.
10. Molina-Montes E, Perez-Nevot B, Pollan M, et al. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast. 2014;23:721-42.
11. Newman LA, Kuerer HM, Hunt KK, et al. Educational review: role of the surgeon in hereditary breast cancer. Ann Surg Oncol. 2001;8:368-78.
12. Mallick S, Benson R, Julka PK. Breast cancer prevention with anti-estrogens: review of the current evidence and future directions. Breast Cancer. 2016;23:170-7.
13. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol. 2016;34:4431-42.
14. Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365-83.
15. Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk-Reducing) Mastectomy. Ann Surg Oncol. 2017;24:375-97.
16. Hawley ST, Jagsi R, Morrow M, et al. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014;149:582-9.
17. Newman LA. Contralateral prophylactic mastectomy: is it a reasonable option? JAMA. 2014;312:895-7.
18. Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264:413-20.
19. Landercasper J, Attai D, Atisha D, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons Consensus Conference. Ann Surg Oncol. 2015;22:3174-83.
20. McLaughlin SA. Surgical management of the breast: breast conservation therapy and mastectomy. Surg Clin North Am. 2013;93:411-28.
21. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
22. Pilewskie M, King TA. Magnetic resonance imaging in patients with newly diagnosed breast cancer: a review of the literature. Cancer. 2014;120:2080-9.
23. Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082-8.
24. Fancellu A, Soro D, Castiglia P, et al. Usefulness of magnetic resonance in patients with invasive cancer eligible for breast conservation: a comparative study. Clin Breast Cancer. 2014;14:114-21.
25. Fischer U, Zachariae O, Baum F, et al. The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol. 2004;14:1725-31.
26. Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26:386-91.
27. Hwang N, Schiller DE, Crystal P, et al. Magnetic resonance imaging in the planning of initial lumpectomy for invasive breast carcinoma: its effect on ipsilateral breast tumor recurrence after breast-conservation therapy. Ann Surg Oncol. 2009;16:3000-9.
28. Shin HC, Han W, Moon HG, et al. Limited value and utility of breast MRI in patients undergoing breast-conserving cancer surgery. Ann Surg Oncol. 2012;19:2572-9.
29. Ko ES, Han BK, Kim RB, et al. Analysis of the effect of breast magnetic resonance imaging on the outcome in women undergoing breast conservation surgery with radiation therapy. J Surg Oncol. 2013;107:815-21.
30. Kim JY, Cho N, Koo HR, et al. Unilateral breast cancer: screening of contralateral breast by using preoperative MR imaging reduces incidence of metachronous cancer. Radiology. 2013;267:57-66.
31. Sung JS, Li J, Costa GD, et al. Preoperative breast MRI for early-stage breast cancer: effect on surgical and long-term outcomes. AJR Am J Roentgenol. 2014;202:1376-82.
32. Yi A, Cho N, Yang KS, et al. Breast cancer recurrence in patients with newly diagnosed breast cancer without and with preoperative MR imaging: a matched cohort study. Radiology. 2015;276:695-705.
33. Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol. 2014;32:392-401.
34. Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563-71.
35. Miller BT, Abbott AM, Tuttle TM. The influence of preoperative MRI on breast cancer treatment. Ann Surg Oncol. 2012;19:536-40.
36. Gonzalez V, Sandelin K, Karlsson A, et al. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: a prospective, randomized, multicenter study. World J Surg. 2014;38:1685-93.
37. Vos EL, Voogd AC, Verhoef C, et al. Benefits of preoperative MRI in breast cancer surgery studied in a large population-based cancer registry. Br J Surg. 2015;102:1649-57.
38. Parsyan A, Moldoveanu D, Balram B, et al. Influence of preoperative magnetic resonance imaging on the surgical management of breast cancer patients. Am J Surg. 2016;211:1089-94.
39. Bansal GJ, Santosh D, Davies EL. Selective magnetic resonance imaging (MRI) in invasive lobular breast cancer based on mammographic density: does it lead to an appropriate change in surgical treatment? Br J Radiol. 2016;89:20150679.
40. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507-15.
41. Buchholz TA, Somerfield MR, Griggs JJ, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32:1502-6.
42. Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014;21:717-30.
43. Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217-23.
44. Barnes DM, Newman LA. Pregnancy-associated breast cancer: a literature review. Surg Clin North Am. 2007;87:417-30.
45. Loibl S, Schmidt A, Gentilini O, et al. Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol. 2015;1:1145-53.
46. Chen AM, Obedian E, Haffty BG. Breast-conserving therapy in the setting of collagen vascular disease. Cancer J. 2001;7:480-91.
47. Varga Z, Mallon E. Histology and immunophenotype of invasive lobular breast cancer: daily practice and pitfalls. Breast Dis. 2008;30:15-9.
48. Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15:1297-303.
49. Newman LA, Kuerer HM. Advances in breast conservation therapy. J Clin Oncol. 2005;23:1685-97.
50. Morrow M. Personalizing extent of breast cancer surgery according to molecular subtypes. Breast. 2013;22(suppl 2):S106-S109.
51. Mota BS, Riera R, Ricci MD, et al. Nipple- and areola-sparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev. 2016;11:CD008932.
52. Headon HL, Kasem A, Mokbel K. The oncological safety of nipple-sparing mastectomy: a systematic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg. 2016;43:328-38.
53. Nash R, Goodman M, Lin CC, et al. State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004-2012. JAMA Surg. 2017 Mar 29. [Epub ahead of print]
54. Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA. 2014;312:902-14.
55. Guth U, Myrick ME, Viehl CT, et al. Increasing rates of contralateral prophylactic mastectomy - a trend made in USA? Eur J Surg Oncol. 2012;38:296-301.
56. Kroll SS, Miller MJ, Schusterman MA, et al. Rationale for elective contralateral mastectomy with immediate bilateral reconstruction. Ann Surg Oncol. 1994;1:457-61.
57. Bloom ND, Daluvoy RV, Ceccarelli F, Degenshein GA. Bilateral mammary carcinoma; immunologic implications. NY State J Med. 1980;80:908-10.
58. Rutqvist LE, Cedermark B, Glas U, et al. Contralateral primary tumors in breast cancer patients in a randomized trial of adjuvant tamoxifen therapy. J Natl Cancer Inst. 1991;83:1299-306.
59. Graham MD, Yelland A, Peacock J, et al. Bilateral carcinoma of the breast. Eur J Surg Oncol. 1993;19:259-64.
60. Robinson E, Rennert G, Rennert HS, Neugut AI. Survival of first and second primary breast cancer. Cancer. 1993;71:172-6.
61. Healey EA, Cook EF, Orav EJ, et al. Contralateral breast cancer: clinical characteristics and impact on prognosis. J Clin Oncol. 1993;11:1545-52.
62. Singletary SE, Taylor SH, Guinee VF, Whitworth PW Jr. Occurrence and prognosis of contralateral carcinoma of the breast. J Am Coll Surg. 1994;178:390-6.
63. Lee JS, Grant CS, Donohue JH, et al. Arguments against routine contralateral mastectomy or undirected biopsy for invasive lobular breast cancer. Surgery. 1995;118:640-7.
64. Mose S, Adamietz IA, Thilmann C, et al. Bilateral breast carcinoma versus unilateral disease: review of 498 patients. Am J Clin Oncol. 1997;20:541-5.
65. Gajalakshmi CK, Shanta V, Hakama M. Survival from contralateral breast cancer. Breast Cancer Res Treat. 1999;58:115-22.
66. Peralta EA, Ellenhorn JD, Wagman LD, et al. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg. 2000;180:439-45.
67. Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst. 2010;102:401-9.
68. Boughey JC, Hoskin TL, Degnim AC, et al. Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol. 2010;17:2702-9.
69. Brewster AM, Bedrosian I, Parker PA, et al. Association between contralateral prophylactic mastectomy and breast cancer outcomes by hormone receptor status. Cancer. 2012;118:5637-43.
70. Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014;348:g226.
71. Li X, You R, Wang X, et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta-analysis and systematic review. Clin Cancer Res. 2016;22:3971-81.
72. Pesce C, Liederbach E, Wang C, et al. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol. 2014;21:3231-9.
73. Yao K, Winchester DJ, Czechura T, Huo D. Contralateral prophylactic mastectomy and survival: report from the National Cancer Data Base, 1998-2002. Breast Cancer Res Treat. 2013;142:465-76.
74. Jatoi I, Parsons HM. Contralateral prophylactic mastectomy and its association with reduced mortality: evidence for selection bias. Breast Cancer Res Treat. 2014;148:389-96.