The passage of the Affordable Care Act in 2010 initiated discussion regarding transitioning from a fee-for-service arrangement of care reimbursement to value-based care. Cost-effectiveness analysis (CEA) has been used in the past to quantify value as it relates to the provision of healthcare. New treatments or techniques being compared with other new or existing therapies or approaches to care were determined to be cost-effective if the incremental cost-effectiveness ratio was less than $50,000/life-year or quality-adjusted life-year. This result was accepted as a proxy for value in care delivery. The calculation of value, however, is the inverse of CEA, with units measured in outcome/cost. Given the wealth of medical information now available online, patients are becoming more sophisticated consumers of healthcare, investigating not only outcomes but also costs of care associated with different treatment approaches. Costs to be considered include direct medical costs; the indirect medical costs associated with treatment; and productivity costs resulting, for example, from time lost from work when patients must travel to a cancer center or clinic to receive treatment. Radiation oncologists must be mindful of these costs when designing treatment plans. Increased adoption of hypofractionated radiation treatment strategies (ie, higher radiation doses given over a shorter course of treatment) could increase patient value by reducing direct and indirect medical costs, as well as productivity costs.
Cost vs Value
With the passage of the Affordable Care Act (ACA) in 2010, increased emphasis has been placed on “value” as it relates to medical care. The ACA established value-based purchasing, with the Centers for Medicare and Medicaid Services (CMS) rewarding hospitals based upon the quality of care provided; on how closely best clinical practices are followed; and on the ways in which hospitals enhance patient experiences of care during their hospital stay, compared with previous reimbursement models. Value-based incentives for hospitals are based upon a combination of clinical care processes, patient and caregiver experiences, efficiency and cost reduction, and clinical and safety outcomes.
How is “value” defined? We all have an idea of the meaning of value as it relates to our daily lives. Cars made in Germany are assumed to have a higher value compared with cars made in Eastern Europe. A “two-for-one” sale has more value than buying a product at the regular price. Value in its essence is preference or outcome divided by cost, or described in terms of a mathematical equation, value = outcome (preference)/cost. It follows, then, that value is increased by holding outcome (preference) constant and reducing cost, or by improving outcome (preference) while holding cost constant-and cost is only half of the value equation.
Although payers may only focus on cost, value can be increased by improvement of outcome or patient preference. To think of value in a more familiar context, value is the inverse of cost-effectiveness-whereas value = outcome/cost, cost-effectiveness = cost/outcome.
Difficulties in Determining Value
Value perspective
The output of the value equation, mathematically defined, can vary depending upon the viewpoint, as will be described subsequently. In addition, value as it relates to medical care is a concept that can be difficult to quantify. While it may be fairly easy to quantify value outside of a hospital or medical setting, patients may not be able to quantify value within the medical system because they do not have accurate information about the two critical aspects of value, namely outcome and cost. In general, patients who are covered by health insurance do not know the “true” cost of care beyond the cost of copayments and deductibles. In addition, cost of care may differ between two competing treatment alternatives that may not include costs related to treating complications or the cost of taking time off from work. Healthcare delivery value can be defined differently depending on the perspective of the viewer-that is, insurers vs patients.
Payer value. Payers view value as the ability of a treatment to improve outcomes while minimizing cost. An acceptable definition of outcomes can be difficult. Do payers consider factors such as overall survival, progression-free survival, or response rates? Outcomes can also be difficult to measure for payers because patients and their families can move between health plans or they can disenroll, thus making it difficult for payers to determine if a treatment has been successful or if the patient has experienced a costly toxicity. A single-payer health system, such as Medicare, would provide the necessary data to determine ultimate outcomes and the ability to compare outcomes between providers.
Patient value. Value as it pertains to patients should be the most important viewpoint, but given the difficulties with outcomes and cost, it may be the most difficult to quantify. Some view value as being defined by the customer.[1] Yet who is the customer in the delivery of healthcare? Is the patient the customer, or is it the payer, who directly reimburses the deliverers of care? Physicians view patients as their “customers,” but what is the reality? A customer in a classic sense is the person paying for a good or service; however, in paying an insurance premium, most patients enter into contracts with payers to negotiate rates of payments. Therefore, with the exception of copays, the final payment to providers is not from patients, but from payers. In addition, patients may have various levels of copays or other out-of-pocket expenses, accounting for differences in the total cost of treatment even for similar treatments given to two different patients. Patients also may have different levels of risk tolerance for the toxic effects of specific agents or treatment approaches, thereby causing differences in outcome if measured by quality-adjusted life-years (QALYs).
One of the limitations acknowledged by the authors of the American Society of Clinical Oncology (ASCO) Value Framework is the lack of inclusion of patient-reported outcomes. Patients may view a certain treatment as having less value if they have higher associated nonmedical costs, such as travel or caregiver costs. Although these costs are not traditionally taken into consideration when calculating the overall cost of care, they are now being recognized as patients become more active consumers in deciding what treatments they are willing to undergo.
Outcome data
Patients may not have sufficient information about provider outcomes to fully inform a decision regarding which treatment-or even healthcare provider-to select. Unlike reports about hospitals from organizations such as the CMS or the Leapfrog Group (with the latter being a national nonprofit consortium of large employers and other healthcare purchasers, founded by the Business Roundtable in 2000 to increase the transparency of reporting about hospital care), standardized reports on outcomes of cancer treatments are not readily available for patients and their families to review prior to making treatment decisions. There are relatively few randomized trials reporting outcomes between two competing treatments utilizing different radiation treatment technologies, let alone a comparison of toxicities.
Cost data
Cost is the second variable in the value equation. The critical question is: what costs should be included and collected? There are direct medical costs (cost of treatment), indirect medical costs (costs such as caregiver costs and costs incurred to attend treatment sessions), and productivity costs (eg, the cost of missing work). The question of which cost to include will depend upon the perspective of the particular group affected. If value is viewed from a payer’s perspective, then only direct medical costs would be included; the payer would not include productivity costs or indirect medical costs. The payer would not care about childcare costs incurred in order for patients or their loved ones to receive treatment. On the other hand, if value is viewed from a patient’s point of view, these costs would need to be included in the value equation, as well as the cost of health insurance premiums. The actual treatment costs would not be of concern to the patients. An analysis of value from a societal viewpoint would include all of these costs. Zheng et al, in reviewing the 2008 to 2012 Medical Expenditure Panel Survey and its Household Component data, found that productivity costs accounted for 39.3%, 32.5%, and 30.8% of medical expenditures for patients with colon, breast, and prostate cancer, respectively, who were between 18 and 64 years of age; in contrast, productivity costs were 30.2%, 25.4%, and 29.9%, respectively, for patients older than 65 years of age.[2]
Cost, like outcomes, would be difficult to measure in the absence of a single payer, since the cost of treatment could become fragmented as patients move between payers. In addition, it is important to consider whether the cost of treating complications should also be included, given that a treatment that may improve clinical outcome may also carry high toxicity costs.
The History of Cost-Effectiveness Analysis (CEA) in Radiation Therapy: A Proxy for, and a Precursor to, Value
CEA has traditionally been the proxy for value, with treatments having cost-effectiveness ratios of less than $50,000 per patient life or QALY being considered of value. Cost-effectiveness studies have been used to justify new and sometimes costly therapies when compared with standard treatments but have never been used to approve or deny treatment, at least in the United States. In certain countries, organizations exist that provide an evaluation of new treatments and can use CEA in their evaluation to determine whether the new treatment should be adopted within the national healthcare system. The National Institute for Health and Care Excellence (NICE) is an independent organization established by the British government in 1999 in England. NICE was created to reduce variation in the availability and quality of drugs, treatments, and care provided by the National Health Services in England and Wales. The organization evaluates new treatments and assesses cost-effectiveness as part of their methodology.
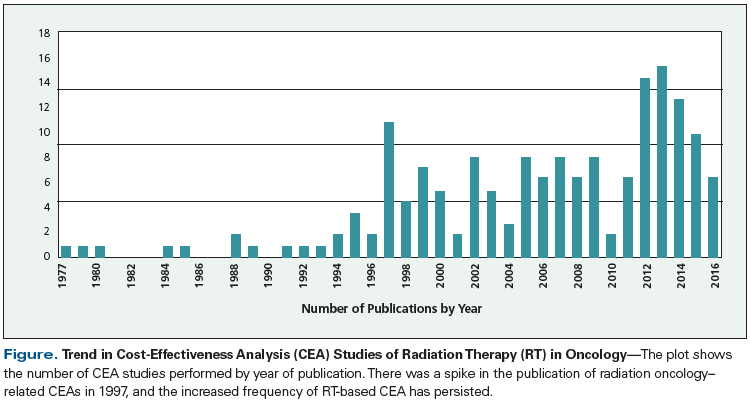
A PubMed search of the Medline database using the terms “cost-effective” and “radiotherapy” retrieved 1,154 unique published studies. When review articles and other types of articles not fitting the criteria for CEA were excluded, 184 studies could be classified as appropriate CEAs. The Figure shows a plot of the number of radiation therapy CEAs by year of publication. There was a spike in the publication of radiation oncology–related CEAs in 1997, which has persisted to the present time period. This interest in performing CEA could have coincided with former President Bill Clinton’s interest in a single-payer healthcare system in 1993, known officially as the Health Security Act and nicknamed “Hillarycare” by its detractors, after former First Lady Hillary Clinton. However, it is most likely that the spike in CEA was a result of the publication of the classic textbook, Cost-Effectiveness in Health and Medicine, edited by Marthe R. Gold and colleagues and published in 1996. The book outlines the recommendations of the Panel on Cost-Effectiveness in Health and Medicine, which was convened by the US Public Health Service to propose guidance on performing CEA in medicine.[3-5]
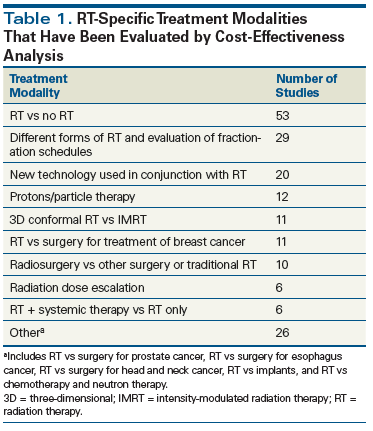
A list of radiation therapy–specific modalities that have been investigated via a CEA is depicted in Table 1. The first published CEA in radiation oncology was an article by Niederer, published in 1977 in the British Journal of Radiology, analyzing the cost-effectiveness of neutron radiotherapy. Notably, the authors wrote, “The alarming rate at which the cost of medicine is growing concerns the medical authorities of most countries more and more.”[6] This is a profound statement, given that it was made in an era with relatively low costs compared with today and prior to the institution of cost-containment mechanisms such as the Diagnosis-Related Groups system in the United States.
CEAs have been performed in oncology to evaluate new management approaches or treatment modalities against the existing standard treatments. The majority of published studies compare various radiation therapy techniques vs existing non–radiation therapy alternatives for the treatment of certain diseases or conditions (see Table 1). Studies published with the greatest frequency were those in patients with breast cancer, in whom the technique of lumpectomy followed by radiotherapy was compared with surgery; and the use of stereotactic body radiation therapy (SBRT) vs lobectomy in early-stage lung cancer. Second in order of frequency were published studies considering multiple approaches to radiation therapy for the treatment of brain metastases, such as various platforms for the delivery of radiosurgery. Third in order of publication frequency were CEAs investigating the outcomes of radiation treatments that included specific technologies, such as tracking devices, compared with radiation treatments that did not include those technologies. A 1979 investigation of the cost-effectiveness of a radiation therapy simulator was the first study published in this category.[7]
It is hard to fathom that a cost-effectiveness study was ever required for a procedure such as CT simulation, which is accepted today as standard of care, yet some institutions continue to evaluate costs and benefits of technology such as positron emission tomography vs CT in diagnosis and staging, and in radiation therapy treatment planning. The fourth most frequent type of CEA of radiation therapy published in the medical literature encompasses studies assessing the benefits of more expensive vs less expensive radiation treatment strategies. Included in this category were studies comparing three-dimensional conformal radiation therapy (3D-CRT) with intensity-modulated radiation therapy (IMRT); or treatment with proton therapy vs non–proton therapy options, usually IMRT. The premise of these analyses is that, while higher costs may be incurred with the use of certain newer technologies, in some cases the added expense of therapy is offset by improved outcomes, such as better tumor control and/or reduced treatment-related toxicity.
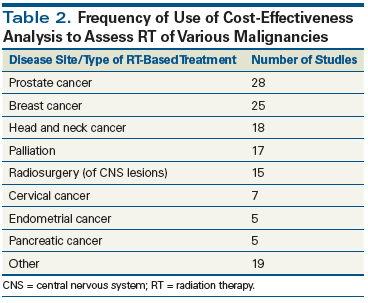
The anatomic sites of various malignancies and the frequency of their evaluation by CEA are detailed in Table 2. It is not surprising that the two most common types of cancer treated by radiation therapy are the two disease sites most commonly studied by CEA. As mentioned previously, CEA is performed to evaluate older vs newer treatments, such as 2D-CRT or 3D-CRT vs IMRT or proton therapy in the management of prostate cancer. CEA is also used to assess new treatment techniques or inclusion of new systemic therapies in patients with head and neck cancer. Given the potential higher costs of radiation therapy, it is not surprising that there were 17 publications evaluating different palliative treatment approaches employing radiation therapy. It is surprising, however, that there were five published CEAs of radiation treatment of pancreatic cancer. A CEA in pancreatic cancer would not fulfill one of the criteria set forth by Hillner and Smith for performing a CEA, because pancreatic cancer is a relatively uncommon malignancy, and standard treatment is so ineffective that clinicians would adopt any type of therapy generating an improvement in outcome.[8]
The quality of selected published CEAs and the legitimacy of their conclusions regarding the value of radiation therapy have been questioned, however. Barbieri et al evaluated radiation therapy CEAs performed in breast, cervical, colorectal, head and neck, and prostate cancers, to determine whether the methodologies used in these analyses conformed to those defined in the guidelines used by the NICE technology appraisal program.[9] A total of 29 studies met the inclusion criteria for systematic review, with a majority of the published studies not meeting the essential UK-specific criteria determined by NICE. Therefore, in order to properly weigh the evidence as a measure of value, the quality of a given analysis must be evaluated with respect to its conclusions.
CEAs have been used as a proxy to define value as it relates to reimbursement. Treatments lower than the accepted threshold for cost-effectiveness have been concluded to be of value and should be adopted and reimbursed. In the United States, CEAs have not been used to determine whether or not a particular treatment should be reimbursed, and the ACA specifically states that CEAs should not be used to determine insurance coverage of treatments. However, the ACA serves as the basis for initiating the conversation about value-based purchasing. CEAs published to date have served as starting points for discussions that should then be transformed into analyses of value. While value and cost-effectiveness are similar in their definitions, as mentioned previously, the methodology does not exist today to accurately calculate value and to compare the value added of various treatments, let alone compare disparate treatments against each other. The processes to perform these analyses will need to be developed in the future.
Recent Attempts to Measure Value in Radiation Therapy
ASCO published its first iteration of a Value Framework in 2015, and an update was published in 2016.[10,11] The ASCO Value Framework focuses on information about the clinical benefit, toxicity, and symptom palliation achieved with various cancer treatment approaches, as derived from comparative trials. To calculate the Net Health Benefit of a particular regimen, scores are combined from data on clinical benefit and toxicity, with bonus points rewarded for a long tail of the survival curve (ie, an extended plateau, suggesting cures), as well as bonuses for symptom palliation, improved quality of life, and longer treatment-free intervals. Cost is also included in the analysis.[10] Determination of the overall cost, however, only includes drug-acquisition costs and patient copays; others that are important to cancer patients and their families-such as the costs of traveling to receive treatment, hospitalizations due to side effects or other complications, lost time from work, and the various costs incurred by caregivers-are not considered. The authors, in their first attempt to construct a value framework, fully realize these and other limitations. The ASCO Value Framework also does not permit cross-trial comparisons, which are important in evaluating two disparate treatments for the same condition.[10]
Similarly, the American Society for Radiation Oncology (ASTRO) offers value guidelines for their membership on radiation therapy delivery. They have created a best practices initiative following accepted RAND processes to help members design guidelines for use in their own institutions.[12] In addition, ASTRO and the Radiation Oncology Institute are investing in research to evaluate ways that radiation oncologists can increase value by assessing QALY as an outcome (ie, cost-utility assessments), analyzing productivity loss for patients and their caregivers associated with alternative approaches, and evaluating comparatively alternative treatments so that individual patients can decide which treatment approach best meets their needs.[12]
Teckie and colleagues from the University of California, Los Angeles have published a framework for radiation oncology by including structure and process along with outcome in the traditional value equation,[13] as outlined previously and by others.[14] Teckie et al argue that there are five challenges to achieving value in oncology care, encompassing structure, process, outcomes, cost, and global domains.
• Elements of the “structure” domain include lack of cross-specialty integration of care, lack of meaningful practice standardization/accreditation, and lack of meaningful practice certification.
• The “process” domain consists of inadequate emphasis on accessibility, timeliness, and coordination of care; provider-centered rather than patient-centered care; and insufficient use of evidence-based guidelines.
• The “outcomes” domain is compromised by limitations such as inadequate systems for handling longitudinal discrete patient data, lack of standardized instruments with which to measure patient-reported outcomes, and inadequate subjective and objective outcomes data to inform quality assurance activities.
• The “cost” domain includes costs that are not measured well with respect to disease process, financial incentives favoring overutilization, and costs that are not transparent and commensurate with quality care.
• Lastly, the “global” domain comprises information asymmetry between patients and providers, overutilization driven by moral hazard and provider-induced demand, and difficulties in risk stratification between patient groups that hinder intergroup comparison.[13]
To address the challenges to value in the structure domain, Teckie et al propose integrated practice unit models for the delivery of care, and promotion of practice accreditation to reduce variation among practices and to engage patients in the selection of providers who meet high-volume standards. To address the considerations related to the process domain, the authors propose optimization of accessibility, timeliness, and care coordination; patient-centered process standards; and facilitation of patient access to information about process aspects of their care. To address issues relevant to the outcomes domain, Teckie and colleagues recommend measurement of objective and subjective outcomes, creation of easy-to-use national registries, and reporting by providers of patient outcomes and provider adherence to guidelines and best practices. Finally, value is also driven by payment reform strategies, including measurement of all costs of treatment related to an episode of care, and knowledge of the true total cost of a cycle of care (ie, beyond billed charges).[13]
Trends in Value in the Delivery of Radiation Therapy
Patients are becoming informed consumers of healthcare and will be seeking practices with a high volume of procedures, such as SBRT, in an attempt to improve outcomes. Patients may also seek out hypofractionated treatment in an effort to reduce the duration of therapy. Indeed, hypofractionated radiation therapy is becoming standard in the treatment of breast and prostate cancers, as well as in palliation of symptoms. However, despite the publication of clinical trials and consensus guidelines on the use of hypofractionated radiation, uptake has varied. For example, the Michigan Radiation Oncology Quality Consortium reported treatment outcomes for patients receiving radiation therapy at 13 practices in Michigan between October 2011 and December 2013. The use of hypofractionated courses of radiotherapy for patients with T1–2N0 breast cancers ranged from 2% to 80%.[15] Hypofractionated courses of therapy would increase value not only by reducing cost but also by improving patient satisfaction and, in turn, patient-reported outcomes. However, the use of hypofractionated radiotherapy could affect a hospital’s “bottom line” by decreasing revenue as a result of fewer daily radiotherapy treatments. A recent report detailed the effects of the increased adoption of hypofractionated therapy on a hospital department using a single linear accelerator. Both the hospital and individual physician would generate less revenue. In addition, the number of radiation therapists required to treat patients could also be reduced, affecting the supply and demand.[16]
The practice of medicine is adapting to the changing healthcare environment. Health system consolidation and mergers between payers are moving practices toward a more coordinated enterprise. In selected cases, electronic medical record systems are allowing better communication between specialists and family physicians, and reducing the duplication of services and thus cost. Theoretically, outcomes will also be improved, with everyone on the healthcare team having almost immediate access to patients’ test information, facilitating care across the continuum. Electronic medical records will also allow for the use of patient portals, potentially improving communication between patients and providers.
KEY POINTS
- Cost and value have become important determinants of care but have been variably studied as they relate to radiotherapy.
- Consideration should be given to inclusion of patient-reported outcomes in the evaluation of new technologies, to prove that a given new technology adds value for patients.
- Patient and caregiver costs should be included in the performance of any cost-effectiveness analysis, to measure both how new treatments would affect patients and the "financial toxicity" of treatment.
The practice of radiation oncology today is much different from how it was performed 5 to 10 years ago. For example, sophisticated treatment planning systems and on-board imaging techniques to improve radiation treatment accuracy have enabled radiation oncologists to determine that shorter course treatments are as effective as longer ones, and toxicity levels are similar to those observed with the more traditional longer courses of therapy. As a further demonstration of patient involvement in their care, patient-reported outcomes have been added to the Common Terminology Criteria for Adverse Events (ie, the PRO-CTCAE).
The Oncology Care Model initiative spearheaded by CMS aims to align financial incentives with improved patient care. Documentation of radiation oncology utilization is one of the quality indicators being measured. However, this may result in an underutilization of appropriate care if medical oncologists perceive that they are referring “too many” patients for appropriate radiotherapy. The denial of appropriate radiotherapy would increase value by reducing cost, but outcomes and patient-reported outcomes could also be decreased, resulting in a zero sum gain.
In summary, patients are taking a more active role in their care and are researching what treatments are available to them. Radiation oncologists will need to take into consideration patient preferences with respect to length of treatment. The amount of time that patients devote to treatment will become a factor in deciding what treatment to undergo; when appropriate, shorter courses of treatment will need to be considered. This will have an effect on radiotherapy departments by decreasing revenue, as well as the need for radiation therapists and radiation oncologists.[16] Decreased patient treatments would increase value for patients by decreasing productivity costs and also by decreasing indirect medical costs. Decreased patient treatments would increase value for payers by decreasing direct medical costs. Increased use of hypofractionated regimens would decrease treatments; with a decrease in the use of radiotherapy to treat cancer, an oversupply of radiation oncologists is projected from 2015 to 2025.[17]
Conclusion: Concrete Steps Oncologists Can Take Now to Increase Value in Radiation Therapy
Radiation oncologists will need to adjust to the changing treatment trends in cancer management, by showing that radiation therapy adds value to treatment regimens. However, the current thinking regarding radiation delivery may bring new opportunities. For example, given that patients with breast and prostate cancers can now undergo shorter courses of high-dose radiation therapy, they may choose hypofractionated radiation treatment over mastectomy or radical prostatectomy, respectively-since they may find a 4- or 5-week course of radiation therapy to be more palatable than the 5- or 8-week course of radiation therapy that has historically been prescribed for the treatment of breast and prostate malignancies, respectively.
Radiation oncology clinical trials must be designed to measure not only survival but also quality-adjusted survival, to demonstrate its added value by improving patient well-being. Data showing that radiation treatments improve outcomes and reduce costs will be needed, to promote the understanding that the addition of radiation therapy to treatment regimens improves value. It will no longer be sufficient to develop treatment techniques or equipment that can improve dose distribution profiles. In order to consider radiation therapy as a treatment that enhances value, improvements in dose distribution will need to produce better clinical outcomes or enhance patients’ quality of life.
Not only should clinical trials be designed to measure response rate and survival, but they should also include important patient-reported outcomes, to show that the treatment or device under investigation adds value as determined by patients. In the current era of cancer management, new systemic agents can be approved by the US Food and Drug Administration if they produce a response to treatment, even if it does not necessarily improve survival or quality of life. Radiation oncology needs to take the lead in designing and conducting trials to prove that value is added-and new devices and treatments should only be approved if they improve value.
Financial Disclosure:The author has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-81.
2. Zheng Z, Yabroff KR, Guy GP Jr, et al. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst. 2016;108:djv382.
3. Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1253-8.
4. Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172-7.
5. Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339-41.
6. Niederer J. A cost-benefit comparative analysis of neutron therapy versus existing radiation therapy techniques in Switzerland. Br J Radiol. 1977;50:923-5.
7. Dritschilo A, Sherman D, Emami B, et al. The cost effectiveness of a radiation therapy simulator: a model for the determination of need. Int J Radiat Oncol Biol Phys. 1979;5:243-7.
8. Hillner BE, Smith TJ. Does a clinical trial warrant an economic analysis? J Natl Cancer Inst. 1998;90:724-5.
9. Barbieri M, Weatherly HL, Ara R, et al. What is the quality of economic evaluations of non-drug therapies? A systematic review and critical appraisal of economic evaluations of radiotherapy for cancer. Appl Health Econ Health Policy. 2014;12:497-510.
10. Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563-77.
11. Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34:2925-34.
12. Mohideen N, Kavanagh BD, Beyer D, et al. Radiation oncology: a perspective on health reform and value-based initiatives. J Oncol Pract. 2014;10:e212-e214.
13. Teckie S, McCloskey SA, Steinberg ML. Value: a framework for radiation oncology. J Clin Oncol. 2014;32:2864-70.
14. Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83:691-729.
15. Jagsi R, Griffith KA, Heimburger D, et al. Choosing wisely? Patterns and correlates of the use of hypofractionated whole-breast radiation therapy in the state of Michigan. Int J Radiat Oncol Biol Phys. 2014;90:1010-6.
16. Konski A, Yu JB, Freedman G, et al. Radiation oncology practice: adjusting to a new reimbursement model. J Oncol Pract. 2016;12:e576-e583.
17. Pan HY, Haffty BG, Falit BP, et al. Supply and demand for radiation oncology in the United States: updated projections for 2015 to 2025. Int J Radiat Oncol Biol Phys. 2016;96:493-500.