The first studies of epidermal growth factor receptor (EGFR) inhibitors in metastatic colorectal cancer were begun before the predictive role of RAS mutations had been elucidated. Secondary analyses of many large randomized trials have shown that mutations in exons 2–4 of KRAS and NRAS, BRAF V600E mutation, and right-sided primary tumor all predict lack of response to EGFR inhibition in the first-line setting. However, even in patient populations defined by a lack of these negative predictors, there is still not uniform response to anti-EGFR therapy. Additionally, although older adults have been shown to have the potential to both tolerate and respond to anti-EGFR therapy, the criteria for selecting the most appropriate older patients for treatment remain unclear.
Introduction
Cetuximab and panitumumab, both monoclonal antibodies targeting the epidermal growth factor receptor (EGFR), were initially developed for use in second-line or subsequent-line treatment of colorectal cancer, administered either as single agents or in combination with irinotecan; the early studies, however, used tumor expression of EGFR as a criterion for treatment eligibility. By the time clinicians had ascertained the importance of RAS mutations in informing the use of anti-EGFR agents in colorectal cancer, studies of these agents in first-line treatment were well underway, and the patterns of care were fairly established. This accounts, at least in part, for the lack of consensus or conviction about when in the continuum of care anti-EGFR agents should be used.
RAS Mutations
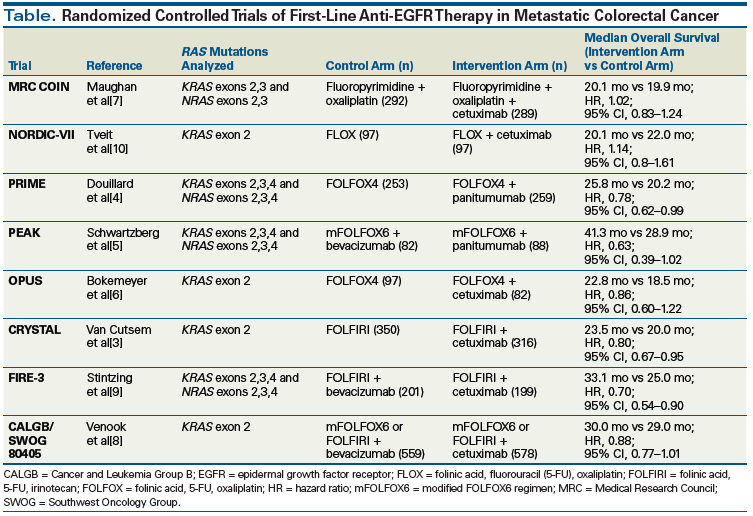
Shortly after EGFR expression was recognized as irrelevant in the management of colorectal cancer (since patients lacking EGFR expression were shown to be able to respond to cetuximab-based therapies),[1] RAS status emerged as an important biomarker in decision making regarding the use of EGFR antibodies.[2] This retrospective finding emerged from the CRYSTAL[3] and PRIME[4] studies of first-line colorectal cancer treatment that included cetuximab and panitumumab, respectively, and which had each enrolled an unselected cohort of patients with metastatic disease. Secondary analyses of both studies[3,4] showed that a virtual lack of benefit of anti-EGFR therapy was correlated with mutations at codons 12 and 13 in KRAS exon 2. However, even with enrichment for KRAS exon 2 wild-type status, the overall response rate in CRYSTAL rose to just 57.3%.[3] This spurred further analyses of other trials of first-line anti-EGFR agents (Table)[3-10] and led to a broadening of the list of activating mutations in KRAS exons that are most predictive of lack of response to these agents.[4,9]
Although proof is lacking that specific mutations in an individual patient absolutely preclude that patient’s ability to respond to anti-EGFR therapy, mutations in KRAS or NRAS exon 2 (at codons 12 and 13), exon 3 (at codons 59 and 61), and exon 4 (at codons 117 and 146) have generally been accepted as biomarkers that predict a lack of response to these drugs. In our practice, RAS mutations outside of these locations are not considered to be contraindications to anti-EGFR therapy, since regarding other mutations as contraindications could exclude patients from receiving potentially beneficial therapy. Similarly, patients with BRAF V600E mutations-although mutations in BRAF, KRAS, and NRAS are mutually exclusive-are also unlikely to benefit from any of the standard anti-EGFR therapy combinations. A large meta-analysis of 10 randomized trials failed to demonstrate a progression-free survival (PFS) or overall survival (OS) benefit for anti-EGFR therapy in BRAF V600E–mutant patients.[11] However, combinations of an EGFR antibody, BRAF inhibitor, and irinotecan have demonstrated activity in those patients.[12] Notably, genomic sequencing of KRAS, NRAS, and BRAF has demonstrated high mutation status concordance between the primary tumor and matched metastatic sites.[13]
Conflicting Data
The Cancer and Leukemia Group B (CALGB)/Southwest Oncology Group (SWOG) 80405 trial was a phase III study of first-line chemotherapy for metastatic colorectal cancer with either FOLFOX (folinic acid, fluorouracil [5-FU], and oxaliplatin) or FOLFIRI (folinic acid, 5-FU, and irinotecan) per the treating physician’s discretion plus either cetuximab or bevacizumab. The study found no difference in OS between the bevacizumab and cetuximab treatment arms, either in the KRAS exon 2 wild-type population or in the subset of patients with extended RAS wild-type tumors.[8] This outcome was somewhat different from the results seen in KRAS exon 2 wild-type patients in the FIRE-3 study, which showed an OS benefit of 28.7 months in patients who received chemotherapy in combination with cetuximab compared with 25 months in those treated with chemotherapy plus bevacizumab (hazard ratio [HR], 0.77; 95% CI, 0.62–0.96). An even greater survival difference was seen in patients with extended RAS wild-type tumors, with OS of 33.1 months in patients who received cetuximab vs 25.6 months in those treated with bevacizumab (HR, 0.70; 95% CI, 0.53–0.92).[14]
Methodologic differences between the CALGB/SWOG 80405 and FIRE-3 studies could account for the disparate results. Notably, the chemotherapy backbones were not the same; FOLFIRI was used in the FIRE-3 study, and in the CALGB/SWOG 80405 study, some patients were treated with FOLFOX and others received FOLFIRI. The findings of MRC COIN-a UK multicenter, randomized, controlled, three-arm trial by the Medical Research Council that evaluated oxaliplatin and fluoropyrimidine vs oxaliplatin and fluoropyrimidine plus cetuximab vs intermittent oxaliplatin and fluoropyrimidine in previously untreated advanced colorectal cancer-suggest that the chemotherapy backbone may have an impact on the efficacy of the EGFR antibodies.[7] The precise clinical scenario may also be important; for example, the New EPOC (Eloxatin Peri-Operative Chemotherapy) trial in the United Kingdom showed that use of cetuximab prior to the resection of isolated KRAS wild-type liver metastases was actually harmful.[15] Similarly, differences in the patterns of care between centers in Europe and the United States introduced variability in patient management after the study-mandated first-line treatment was completed, which had effects on outcomes.
Beyond RAS
A secondary analysis of the all-RAS wild-type patients in the CALGB/SWOG 80405 study showed that, regardless of the treatment arm, right-sided colorectal tumors were associated with a much worse prognosis, with an OS of 21.9 months vs 35.2 months in patients with left-sided tumors (HR, 0.72; 95% CI, 0.56–0.92). In patients with left-sided primary tumors (splenic flexure to rectum), those treated with chemotherapy plus cetuximab had an improved OS of 39.3 months compared with 32.6 months for those who received chemotherapy plus bevacizumab. In patients with right-sided tumors (cecum to hepatic flexure), the use of bevacizumab was actually associated with an improved OS of 29.2 months compared with 13.6 months with cetuximab.[16] Perhaps most notably, analysis of the FIRE-3 trial by tumor sidedness showed results concordant with those of CALGB/SWOG 80405. Cetuximab yielded a significant survival advantage over bevacizumab when each was administered in combination with FOLFIRI to patients with left-sided tumors (OS, 38.3 months vs 28.0 months, respectively). However, a trend towards benefit was seen with administration of bevacizumab for the treatment of right-sided tumors (OS, 23.0 months vs 18.3 months with cetuximab; HR, 1.44; 95% CI, 0.81–2.11).[17]
US and European investigators performed a retrospective pooled analysis[18] of patients with KRAS/NRAS wild-type metastatic colorectal cancer from five trials of anti-EGFR agents in first-line therapy (CRYSTAL, FIRE-3, PRIME, PEAK, and CALGB/SWOG 80405) and one trial of second-line therapy, the 20050181 randomized phase III study of FOLFIRI with or without panitumumab.[19] Because patients were not randomized based on sidedness, in most of these trials there were baseline differences between patients with left-sided vs right-sided primaries. Specifically, in some studies, patients with left-sided primaries more often had either liver-limited or liver-dominant disease. In the pooled analysis of all six studies, a significantly worse prognosis was observed for patients with right-sided tumors; the HR for OS in right-sided vs left-sided tumors was 1.38 (95% CI, 1.17–1.63). In left-sided tumors, treatment with anti-EGFR therapy plus chemotherapy was associated with improved OS compared with the control arm of chemotherapy with or without bevacizumab (HR, 0.75; 95% CI, 0.67–0.84). However, no benefit of EGFR antibody treatment was seen in patients with right-sided tumors (HR, 1.12; 95% CI, 0.87–1.45).
Although ideally a randomized trial would address the role of anti-EGFR therapy in right-sided tumors, the aforementioned negative findings make such a trial almost unethical, and National Comprehensive Cancer Network guidelines now recommend that EGFR inhibitors be used only in patients with left-sided primary tumors.[20] The preponderance of evidence is such that we do not recommend anti-EGFR therapy in the first line for our patients with right-sided colorectal tumors, except in combinations that include a BRAF inhibitor in BRAF V600E–mutated patients, as in the study by Kopetz et al.[12] The data do not preclude benefit of EGFR antibodies in patients with right-sided colorectal tumors treated beyond first-line therapy, so the optimal approach to this clinical scenario remains an open question. The National Cancer Institute of Canada CO.17 trial compared single-agent cetuximab against best supportive care in chemotherapy-refractory metastatic colorectal cancer, and a PFS advantage was found in KRAS wild-type patients with left-sided, but not right-sided, tumors.[21] However, given that this was a single trial and used only single-agent cetuximab, we do not feel that the evidence is conclusive enough to rule out use of anti-EGFR therapy in right-sided tumors in later lines of treatment.
KEY POINTS
- Tumor molecular profiling should be performed on all patients newly diagnosed with metastatic colorectal cancer, and should include testing for mutations in KRAS and NRAS exons 2–4 and BRAF.
- We do not recommend the use of epidermal growth factor receptor (EGFR) inhibitors in the first-line treatment of right-sided metastatic colorectal cancer.
- Age alone should not be considered a contraindication to anti-EGFR therapy.
Patient Age
The use of anti-EGFR agents in older adults is appealing due to the decreased risk of neutropenia, nausea, and vomiting associated with their use, compared with cytotoxic chemotherapy. However, these targeted agents carry their own set of risks, particularly rash and diarrhea. The risk of diarrhea is of particular concern in older adults, because of their increased potential for dehydration, kidney injury, and electrolyte disturbances. Due to the frequent exclusion of older patients from clinical trials, data are very limited regarding both the efficacy of anti-EGFR agents and the occurrence of these adverse effects in this population. A subset analysis of patients aged 65 years and older in the PRIME study found that the addition of panitumumab to FOLFOX improved OS but yielded no PFS advantage compared with FOLFOX alone.[22] In a retrospective study of 56 heavily pretreated patients aged 70 years and older with metastatic colorectal cancer, both the rate of response to treatment with cetuximab and irinotecan (21.4%) and the level of treatment toxicity were similar to outcomes reported in younger patients.[23] In addition, a retrospective study of 117 patients 65 years of age and older treated with anti-EGFR therapy, either alone or in combination with cytotoxic chemotherapy, found response rates and toxicity profiles comparable to those reported in younger patients, with no association between age and the occurrence of grade 3 or higher toxicity.[24] A prospective nonrandomized study comparing patients younger vs older than age 65, with metastatic colorectal cancer treated with irinotecan with or without cetuximab, found similar levels of efficacy and toxicity between the two age groups.[25] In our practice, we offer anti-EGFR therapy to older adults as part of combination chemotherapy, in combination with irinotecan, or as monotherapy, depending on patient fitness and comorbidities.
Conclusion
Anti-EGFR therapy is a valuable addition to the armamentarium of treatment options for patients with metastatic colorectal cancer. However, RAS mutation status is an imperfect biomarker for prediction of therapeutic outcomes in this setting. The recent discovery of tumor sidedness as a predictor of response highlights how little we understand about which patients are the most appropriate to receive drugs that target EGFR, and at what point in cancer treatment these agents are best used. The current studies evaluating anti-EGFR therapy in combination with BRAF-targeted therapy are a perfect example of the type of work needed to help the largest number of patients benefit from these types of drugs in the future.
Financial Disclosure:Dr. Venook receives research funding from, and is an advisor to, Genentech/Roche; receives research funding from Merck KGA; and serves as an advisor to Taiho. Dr. Ursem has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005; 23:1803-10.
2. Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-34.
3. Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-9.
4. Douillard JY, Oliner KS, Siena S, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013; 369:1023-34.
5. Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-7.
6. Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011; 22:1535-46.
7. Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-14.
8. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer. JAMA. 2017;317:2392-401.
9. Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426-34.
10. Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-62.
11. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587-94.
12. Kopetz S, McDonough SL, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol. 2017;35(4 suppl):abstr 520.
13. Janakiraman M, Vakiani E, Zeng Z, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res. 2010;70:5901-11.
14. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-75.
15. Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601-11.
16. Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15 suppl):abstr 3504.
17. Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer. JAMA Oncol. 2017;3:194-201.
18. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol. 2017;28:1713-29.
19. Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-13.
20. National Comprehensive Cancer Network. NCCN guidelines. Colon cancer. Version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed October 13, 2017.
21. Brulé SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405-14.
22. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study.
J Clin Oncol. 2010;28:4697-705.
23. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol. 2008;67:255-62.
24. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti–epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer. 2014;13:192-8.
25. Jehn CF, Böning L, Kröning H, et al. Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer. Br J Cancer. 2012;106:274-8.