Diagnosis and Treatment of Thrombocythemia in Myeloproliferative Disorders
Myeloproliferative disorders originate in the clonal expansion of a transformed pluripotential hematopoietic progenitor cell. This results in a group of syndromes that include polycythemia vera, essential thrombocythemia,
ABSTRACT: Myeloproliferative disorders originate in the clonal expansion of a transformed pluripotential hematopoietic progenitor cell. This results in a group of syndromes that include polycythemia vera, essential thrombocythemia, chronic myelocytic leukemia, and agnogenic myeloid metaplasia. Diagnostic criteria for polycythemia vera and essential thrombocythemia were codified by the Polycythemia Vera Study Group in 1967 and 1977. Subsequent modifications include criteria for evidence of clonal proliferation by abnormal bone marrow karyotype and demonstration of erythropoietin-independence of erythropoiesis or reduced serum erythropoietin. Phlebotomy is the mainstay of treatment for polycythemia vera. The defining characteristic of essential thrombocythemia is a sustained elevation of the platelet count above 600,000/mL in an untreated patient. Symptoms and risk factors are the main determinants of treatment options for patients with essential thrombocythemia. High-risk patients are candidates for cytoreduction, whereas lower-risk patients receive either no treatment, low-dose aspirin, or another antithrombotic therapy. The availability of newer nonleukemogenic and megakaryocyte-specific agents warrants a reassessment of current treatment options. [ONCOLOGY 15(8):989-1008, 2001]
Introduction
Myeloproliferative disorders are chronic malignant conditions that originate in the clonal expansion of a pluripotential hematopoietic precursor cell. This proliferating progenitor retains its ability to self-renew, commit, differentiate, and mature to produce increased numbers of functioning cells of the hematic trilineage (ie, red blood cells, white blood cells, and platelets).[1,2] The resulting group of syndromes that comprise chronic myeloproliferative disorders include polycythemia vera, essential thrombocythemia, chronic myelocytic leukemia (CML), and agnogenic myeloid metaplasia.
Chronic myelocytic leukemia meets some of the criteria of a chronic myeloproliferative disease. Because CML is more homogeneous in its features, is uniquely associated with a particular chromosomal abnormality, has a relatively short chronic phase, and almost invariably progresses to acute leukemia within a decade, it is best treated as distinct from a non-CML myeloproliferative disorder. Myelofibrosis is an epiphenomenon in myeloproliferative disorders that occurs most often in agnogenic myeloid metaplasia and may complicate late-stage essential thrombocythemia or polycythemia vera. Its etiology and pathophysiology are poorly understood, and it is both unpredictable and resistant to treatment.[1]
The non-CML myeloproliferative disease syndromes are more heterogeneous than CML, and "hybrid" phenotypes are common and diagnostically challenging. The phenotype determines both the pathophysiology and management of each syndrome during the long period of chronic, indolent proliferation. When appropriately treated, survival is measured in decades. Both essential thrombocythemia and polycythemia vera have a predictable but low incidence of transformation to acute leukemia or myelodysplastic disease. These tendencies increase with the use of potentially leukemogenic drug therapy.[3]
Diagnosis of Polycythemia Vera
TABLE 1

Primary and Secondary Criteria That Establish a Diagnosis of Polycythemia Vera
The sine qua non for the diagnosis of polycythemia vera is erythrocytosis, but the diagnosis also requires demonstration of the involvement of at least one additional cell type of the hematic trilineage. Approximately 50% of cases involve thrombocytosis. The Polycythemia Vera Study Group (PVSG) first codified diagnostic criteria in 1967.[4-6] No single marker is diagnostic for polycythemia vera, so the PVSG chose major and minor criteria that, in certain combinations, establish the diagnosis.[7] The principal subsequent modifications include the addition of an abnormal marrow karyotype as a clonality marker to the primary diagnostic criteria and the demonstration of erythropoietin-independent burst-forming unit growth or reduced serum erythropoietin as minor criteria.[8,9] The modified criteria are listed in Table 1. The inclusion of bone marrow histopathology has been proposed by the European Working Group on Myeloproliferative Diseases.[10] The robustness of histopathological criteria will depend on the definition of morphological changes that are without significant inter- or intra-observer error.
Because the kidneys release erythropoietin in response to hypoxic signals, elevated serum levels of erythropoietin appear in secondary erythrocytosis. Erythroid proliferation in polycythemia vera is not driven by erythropoietin, however, and serum levels are typically low. To date, no specific genetic marker has been associated with polycythemia vera,[10] although evidence of a familial incidence has been accrued.[11,12]
It is essential that any occult bleeding and/or iron deficiency that might mask polycythemia vera be corrected before red blood cell mass is measured.[13] A hematocrit of 60% or greater is always indicative of polycythemia vera if other criteria are met, so the need to measure the red blood cell mass directly can be omitted for patients with hematocrit measurements at this level.[14]
TABLE 2

Characteristics of Each Stage of Polycythemia Vera
Wasserman has suggested that the natural history of polycythemia vera may consist of five stages (Table 2).[15] Only about 15% of patients are diagnosed in stage 1.[9] Michiels has proposed criteria for distinguishing the stages as a patient’s symptoms progress.[9] These distinctions involve progressive changes in the primary and secondary criteria, including findings on bone marrow biopsy, with gradation of myelofibrosis as well as cellularity and megakaryocytes. The characteristic features of bone marrow sections of untreated polycythemia vera patients include increased cellularity due to hyperplasia of all marrow elements and an increase in enlarged megakaryocytes with hyperploid nuclei.[9]
Diagnosis of Essential Thrombocythemia
In 1977, the PVSG defined the original essential thrombocythemia diagnostic criteria and subsequently revised them.[14] The defining characteristic is sustained elevation of the platelet count above 600,000/µL in an untreated patient. In approximately 50% of cases, patients also have elevated white cell counts.[6] Essential thrombocythemia is a diagnosis of exclusion, requiring the requisite platelet count, laboratory evidence excluding other myeloproliferative disorders, and a clinical evaluation finding no cause for reactive thrombocytosis.[14] More specific criteria based on laboratory findings-eg, increased platelet size and heterogeneity, ratio of adenosine triphosphate to adenosine diphosphate, and spontaneous growth of erythroid or megakaryocytic colonies in vitro-were excluded from the revisions because they are not uniformly present in patients with essential thrombocythemia.
TABLE 3

Revised Diagnostic Criteria for Essential Thrombocythemia
Although understanding of the molecular biology of myeloproliferative disorders has advanced, no genetic lesion has thus far been associated with essential thrombocythemia. A clonal karyotypic anomaly (5q-) was identified, but it appears in no more than 5% of patients with essential thrombocythemia.[14] The human megakaryocyte growth and development factor thrombopoietin (TPO) was cloned, and the TPO receptor c-Mpl has been characterized. Studies have shown that expression of c-Mpl protein and m-RNA is markedly reduced in the platelets of patients with essential thrombocythemia. Hepatic production of TPO is signal independent, and serum levels are determined primarily by the efficiency of c-Mpl binding and clearance. In polycythemia vera[16] and essential thrombocythemia patients,[17] serum TPO is normal to elevated, suggesting that an intrinsic defect of c-Mpl transcription may cause decreased receptor expression and, consequently, ineffective TPO clearance. In view of the apparent lack of specificity, the diagnostic value of TPO and c-Mpl assays remain to be determined. The revised (1997) diagnostic criteria for essential thrombocythemia are shown in Table 3.
If the measurements in criterion 3 (iron in marrow, serum ferritin, or red blood cell mean corpuscular volume) suggest iron deficiency, polycythemia vera cannot be excluded unless a trial of iron therapy fails to raise the red blood cell mass into the polycythemic range. A normal or elevated serum ferritin level along with a normal red blood cell mean corpuscular volume effectively excludes both reactive thrombocytosis secondary to iron deficiency and the possibility of polycythemia vera masked by iron deficiency. Furthermore, a hematocrit of 60% or greater is invariably indicative of polycythemia vera and excludes essential thrombocythemia.[14]
Clinical Course and Complications of Polycythemia Vera
Polycythemia vera is a relatively rare disorder, with an annual incidence of 1 to 3 cases per 100,000 population.[6] According to reports, it occurs least frequently in Japan and most frequently in Sweden and among Ashkenazim in northern Israel.[8] The median age of onset is approximately 65 years, but the disorder is occasionally seen in younger individuals. Median survival for patients whose disorder is not effectively controlled is reported to be 1.5 to 3 years.[13] Among those who receive appropriate treatment, median survival is extended to 10 years or more, depending on age at diagnosis.[18]
As with other myeloproliferative disorders, the clinical manifestations of polycythemia vera are determined by the degree of proliferation of the pluripotential hematopoietic precursor cell (PHPC) progenies. The principal complication is thrombosis resulting from increased proliferation of erythrocytes and platelets. Blood viscosity increases exponentially as the hematocrit exceeds 50%.[19] Platelets in polycythemia vera patients show increased adhesiveness and aggregability.[13] This explains the frequency of thromboembolic events in untreated patients with polycythemia vera, with incidence varying among several series studied.[20]
One series found that 14% of essential thrombocythemia patients experienced thromboembolic events prior to diagnosis, and in 20%, such events were the presenting symptoms of the disorder.[18,21] Similar events develop in an additional 30% during the course of treatment.[6] The most common risk factors for vascular complications of polycythemia vera are age and previous history of vascular events. The greatest risk of thrombosis occurs in the first 3 years following diagnosis in patients treated with phlebotomy.[5]
With moderate elevations of viscosity, stasis develops most frequently in the small vessels of the digits, vestibular apparatus, and retinae and may be experienced as erythromelalgia, vertigo, or scotomata.[22] Because of the reversibility of platelet aggregation, these symptoms are usually transient. At higher levels of viscosity and thrombocythemia, however, involvement of arterial circulation may result in transient ischemic attacks, angina, or bowel ischemia. In one series, cerebral ischemia accounted for 70% of arterial thromboses at diagnosis and was as prevalent as myocardial infarction (30%) prior to diagnosis of polycythemia vera.[18,21]
These extreme events are most common in older patients with underlying atherosclerotic conditions.[13] Patients with uncontrolled polycythemia vera are also prone to deep venous thrombosis of the extremities and the hepatic, portal, and splenic veins.[13] Arterial and venous thromboses occur with about equal frequency in polycythemia vera patients. Hemorrhage, the second most common complication of polycythemia vera, develops in approximately 20% of uncontrolled patients.
The principal long-term risk associated with polycythemia vera among patients who survive thromboembolic and hemorrhagic events is progression to stage IV, or postpolycythemic myeloid metaplasia. This condition has a profile indistinguishable from agnogenic myeloid metaplasia. It is reported that 10% of patients transform in approximately 15 years, and 50% after 20 years, regardless of treatment.[23] Approximately 2% of long-term survivors of polycythemia vera progress to stage V, acute myelocytic leukemia.[24]
Clinical Course and Complications of Essential Thrombocythemia
About one third of essential thrombocythemia cases are discovered coincidentally by automated platelet count during routine blood screening. The remaining two thirds are diagnosed in symptomatic individuals who may present with vascular occlusive disturbances such as migraine-like headache or dizziness, thrombohemorrhagic manifestations, neurologic symptoms including transient ischemic attacks, stroke, visual disturbances, and seizures[25], or cutaneous manifestations such as hematomas and ecchymoses.[26] In addition, essential thrombocythemia is one of the rare causes of angina pectoris and myocardial infarction in adults without atherosclerosis.[27] Splenomegaly is less pronounced in essential thrombocythemia than in polycythemia vera.[6]
Essential thrombocythemia is the most common of the myeloproliferative disorders in the United States, with an annual incidence of approximately 2.5 per 100,000 population.[28] It is slightly more common in women than in men. The median age of onset is approximately 60 years, but between 10% and 25% of patients are less than 40 years old. Younger patients present the more challenging management problems because of the complications of long-term treatment.
Although essential thrombocythemia is comparatively benign compared to the other myeloproliferative disorders, there is a high morbidity rate from vascular complications, and quality of life is diminished for patients who experience severe acute or repetitive events.[29] It has been estimated that between 20% and 30% of essential thrombocythemia patients undergo one or more major events.[29] The others may be symptomatic from minor occlusive phenomena in small, medium, or large arteries but develop deep venous thrombosis less frequently.[1]
Hemorrhage occurs less often than thrombosis, and usually in patients with platelet counts in excess of 1,500,000/µL. [1,29,30] The incidence of venous thrombosis is lower in essential thrombocythemia than in polycythemia vera. Age, history of vascular complications, and duration of thrombocytosis are the principal risk factors. While the frequency of events has not been conclusively correlated to platelet level, maintenance of counts of 600,000/µL or below is, by consensus, the critical measure of effective prevention. Nonetheless, thrombotic complications have been reported in patients with counts below this level and even below 400,000/µL. Based on one recent series, authors have recommended that treatment in these patients be aimed at reducing platelet levels to the low end of the normal range.[31]
Approximately 5% of essential thrombocythemia patients progress to polycythemia vera.[6] For 2% of patients, the long-term natural course is to an agnogenic myeloid metaplasia-like profile with life-threatening myelofibrosis and myeloid metaplasia of the spleen.[6,32] Although progression to acute myelocytic leukemia is rare among untreated patients, it occurs in 4% to 5% of those who undergo long-term myelosuppressive cytoreduction. This suggests that while essential thrombocythemia is a progressive preleukemic syndrome, this potential is activated by myelosuppression. It may also increase the probability of evolution to myelodysplastic syndromes.[3]
Therapy
In this section, general treatment considerations for essential thrombocythemia and polycythemia vera are first reviewed individually, and then issues pertaining to contemporary pharmacologic therapy are explored.
Treatment of Essential Thrombocythemia
A patient’s symptoms and risk factors, including age, are the main determinants of treatment options for essential thrombocythemia. An older patient, especially one with a history of vascular events or predisposing atherosclerosis, is almost invariably a candidate for cytoreduction. In this patient, the risk of iatrogenic leukemic transformation is low because of the length of time associated with transformation.
Younger patients, both symptomatic and asymptomatic, present complex dilemmas. Until recently, the asymptomatic younger patient with a history negative for vascular complications was generally considered a candidate for no treatment, low-dose aspirin, or another antithrombotic therapy, but generally not for cytoreduction. In contrast, the high-risk or symptomatic younger patient was considered a candidate for cytoreduction, but with the trepidation that the mechanism of action of some myelosuppressive therapy posed a significant risk for acute leukemic transformation after years of treatment. New therapeutic agents, discussed below, offer more options with the yet unsubstantiated hope of significantly less toxicity.
Treatment of Polycythemia Vera
Because the primary therapeutic target in polycythemia vera is red blood cell mass, early treatment options differ from those for essential thrombocythemia. Phlebotomy (or venesection) is the mainstay of treatment, with the goal of maintaining the hematocrit at 45% in male patients and 42% in female patients. These levels minimize blood viscosity without compromising cerebral blood flow.[33-37] Phlebotomy is the preferred treatment for young women who are at low risk for vascular events but high risk for pregnancy, and in whom myelosuppressive therapy should be avoided if possible.[13] Phlebotomy can be performed as often as twice weekly at the outset and at intervals of 1 to 3 months thereafter for maintenance. It is effective because of the long life of the red cell, and is not applicable to other hematic elements. It results in an absolute reduction in the number of circulating red cells and also induces iron deficiency as a means of inhibiting erythropoiesis.[13]
Low-dose aspirin is often administered to phlebotomy patients for its contribution to thrombus prevention by reducing platelet aggregation. However, the positive effects of aspirin need to be balanced against the increased risk of gastrointestinal bleeding. Allopurinol may also be added as prophylaxis against hyperuricemia-induced gout or renal stones that may accompany increased hematopoietic cell turnover and may be further aggravated by phlebotomy.[13]
There is a high prevalence (30% to 50%) of thrombocythemia among patients with polycythemia vera, and repeated phlebotomy stimulates platelet production. The first PVSG trial found that phlebotomy patients have an increased risk of thrombosis during the first 3 years of treatment (note that the initial hematocrit control level in this study was 52%) and that the incidence of thrombosis correlates, in part, with the frequency of phlebotomy.[4] These observations suggest that phlebotomy should be accompanied by cytoreductive therapy directed at the PHPC.[13]
With the conventional myelosuppressive armamentarium, this once again introduces the risk of leukemic transformation. Consequently, the PVSG advised that low-risk patients undergo phlebotomy alone and that only high-risk patients undergo phlebotomy plus myelosuppression. Based on short-term efficacy and safety studies, the use of hydroxyurea was recommended. The availability of newer, presumably nonleukemogenic agents and megakaryocyte-specific agents has prompted a reassessment of these alternatives.
Contemporary Pharmacologic Treatment
The history of drug development for essential thrombocythemia and polycythemia vera has been one of improving therapeutic efficacy while reducing leukemogenic, carcinogenic, and mutagenic potentials.[38]
Radiophosphorus and Alkylating Myelosuppressive Agents
Radioactive phosphorus (P-32) was introduced for the treatment of myeloproliferative disorders a half century ago and showed remarkable efficacy in reducing both thrombocytosis and erythrocytosis. For example, 98% of patients with polycythemia vera achieved a complete remission after P-32 treatment-in most cases, after a single dose. However, the isotope was also found to be associated with a high incidence of hematologic and nonhematologic malignancies.[4,39] Furthermore, the frequency of acute leukemia and myelodysplastic syndromes in polycythemia vera patients treated with P-32 was determined to be dose-related, increasing linearly beginning with year 5.[39]
In the 1970s, standard treatment consisted of P-32 and/or alkylating myelosuppressive agents such as chlorambucil (Leukeran), busulfan (Busulfex, Myleran), melphalan (Alkeran), and pipobroman (used principally in Europe). Like P32, the alkylating agents are nonspecific myelosuppressives that were found to be efficacious in reducing thrombocythemia and erythrocythemia in clinical trials. Between 1967 and 1978, the European Organization for the Research and Treatment of Cancer randomized 293 previously untreated polycythemia vera patients to P-32 or busulfan and found that busulfan significantly outperformed radiophosphorus with respect to hematologic control, thrombotic morbidity, disease-related mortality, and 10-year overall survival. There were two cases of acute leukemia in the radiophosphorus arm and three in the busulfan arm.[4,9]
The 1968-1974 PVSG study randomized 411 polycythemia vera patients to phlebotomy, radiophosphorus, or chlorambucil. At the end of the followup period, investigators reported a 10.6% incidence of acute leukemia in the chlorambucil arm, an 8.3% incidence in the P-32 arm, and a 1.5% incidence in the phlebotomy arm.[4] It was recommended that use of chlorambucil be discontinued.
Hydroxyurea
Hydroxyurea was introduced in 1977, when the PVSG initiated a trial in 51 myelosuppressive naive polycythemia vera patients. Supplementary phlebotomy was used as necessary to control hematocrits below 45%. Excellent control of hematologic and thrombotic events was achieved throughout a median followup of 9.6 years and a maximum of 15.3 years.[40,41] Hydroxyurea’s efficacy in controlling platelet counts and thrombohemorrhagic complications has also been demonstrated in patients with essential thrombocythemia.[41,42]
Since 1980, hydroxyurea has been the agent of choice for the treatment of thrombocytosis and erythrocytosis due to essential thrombocythemia and polycythemia vera. It is a potent nonalkylating, nonspecific myelosuppressive agent with potential for dose-related cytoreduction of all myelogenous hematic components.[29] Its mechanism of action is the inhibition of DNA synthesis (by blocking the activity of the enzyme ribonucleoside reductase). Because it is a nonalkylating molecule, its use was greeted with the hope that its efficacy would not be offset by leukemogenicity. Those hopes have been largely unrewarded. Long-term safety studies have reported acute leukemic progression rates of 5.9% to 10% and higher.[41,43] A French study comparing the long-term safety risks of hydroxyurea and pipobroman in 292 polycythemia vera patients reported a 10% acute leukemia prevalence for each agent at 13 years and a 15% cancer risk at 14 years (annual incidence: 1.2%).[44]
Trial results regarding leukemic transformation after treatment with hydroxyurea for essential thrombocythemia are similar. The PVSG reported a 21.6% predicted probability of developing acute leukemia at 10 years, with the greatest risk occurring after year 5.[14] A French study in 357 patients compared transformation rates after hydroxyurea monotherapy with those following other myelosuppressive therapies and discontinuation of hydroxyurea. Acute leukemic transformation was significantly higher for all agents used after hydroxyurea, suggesting that hydroxyurea sensitizes myelogenous tissue and potentiates the leukemogenicity of other agents.[3,14] There is no evidence, however, of increased risk for leukemia when hydroxyurea is taken subsequent to withdrawal from other drugs.[14]
In light of its leukemogenicity, hydroxyurea is inappropriate for young patients, low-risk patients, and women of childbearing age. Moreover, the agent is associated with other side effects that may limit its use. These include megaloblastic erythropoiesis, aphthous stomatitis, gastric pain, diarrhea, leg ulcers that require withdrawal, dry skin, alopecia, increases in hepatic enzymes, and neurologic toxicity.[9]
In many patients, achieving adequate platelet control is difficult, except at doses that may induce oversuppression of red cells or neutrophils, making it also difficult to achieve stable maintenance. This situation is further complicated by rebound thrombocytosis that occurs with a reduction in dose or withdrawal of hydroxyurea.
The introduction of a new lower-dose formulation of hydroxyurea (Droxia) may facilitate management with monotherapy and create opportunities for combination therapy when single-agent treatment is inadequate.[38]
Interferon-Alpha
Following reports of the beneficial use of recombinant interferon-alpha (Intron A, Roferon-A) in chronic myelocytic leukemia and demonstration of its selective effect on the malignant clone, Gilbert[45] and Silver[47,48] reported its potential efficacy in polycythemia vera. The interferons are naturally occurring cytokines that modify biological response and modulate the immune system.[29] Interferon-alpha suppresses the proliferation of both pluripotent and lineage-committed hematopoietic progenitors and antagonizes the action of platelet-derived growth factor (produced by megakaryocytes), which mediates fibroblast proliferation.[20] By virtue of these actions, it can induce hematologic and cytogenic remissions in patients with myeloproliferative disorders and is uniquely able to reduce splenomegaly and reverse the process that produces myeloid metaplasia and myelofibrosis.[13,20,45,46] Thus, the foremost benefits of interferon-alpha therapy are seen in patients with polycythemia vera and post-polycythemia vera myeloid metaplasia.[13]
Clinical Trials: No long-term prospective trials comparing interferon-alpha with conventional therapies for essential thrombocythemia and polycythemia vera have been published to date, but several single-arm studies have been reported. One review of 11 such trials encompassing 212 patients with essential thrombocythemia calculated a response rate of 90%, although sustained unmaintained remissions occurred in only 12% of patients.[38,49]
A similar review of nine studies involving 185 patients reported a response rate of 86%. Remission of thrombocythemia was achieved at a median of 12 weeks (range: 3 to 26 weeks). Time to maximum hematologic remission correlated with the dose of interferon and the initial platelet count. Control of complications and symptomatic improvement occurred in 60% to 100% of patients among the studies reviewed. Long-term remission without maintenance therapy was observed in 7% of patients, but most study patients receiving low-dose maintenance therapy achieved long-term remission.[20]
Trial results in polycythemia vera were similar. A review of nine studies encompassing 49 subjects indicated a 60% complete response rate to interferon-alpha therapy and a 23% partial response rate, with complete response defined as not needing phlebotomy to maintain a hematocrit of 45%. A reduction in the size of the spleen and relief from pruritus were observed in 75% of patients. A maximum response was reported in 76% of patients within 6 months of initial treatment (range: 3 to 12 months). A study in 11 patients reported no thrombohemorrhagic events within a mean follow-up of 36 months.[50]
Safety: Interferon is not teratogenic because it does not cross the placenta.[20] Leukemogenicity has not been reported. Hydroxyurea, in contrast, is both teratogenic and leukemogenic. These important differences make interferon-alpha preferable for the treatment of younger patients with myeloproliferative disorders. The issue of treating pregnant women and women who wish to conceive remains cloudy, however. On one hand, it is thought that effective treatment prior to pregnancy-especially if followed by short-term remission through the first trimester-is safe, particularly compared to the use of hydroxyurea, alkylating agents, and radiophosphorus. Intermittent therapy for recurrent thrombocythemia during pregnancy may also be safe, because interferon does not appear to harm the fetus.[1,13] On the other hand, there are insufficient data on which to base such a recommendation. Consequently, manufacturers of interferon-alpha advise against its use during pregnancy.[20]
There are side effects from interferon that affect approximately 30% of patients and have forced 12% to 20% to withdraw from clinical trials.[9] Most common among them, and the one responsible for most trial withdrawals, is a flu-like syndrome that affects the majority of patients taking interferon-alpha. For most patients, this syndrome abates within 1 to 3 months, but for others, it persists.
Other side effects that most frequently result in trial withdrawals are involuntary weight loss, confusion, depression, myalgia, and increased pruritus. Because it is an immune system modulator, interferon may induce autoimmune disorders, especially autoimmune thyroiditis.[20] Also, because side effects are dose-limiting factors, preemptive acetaminophen, night-time administration, and an initial dose equal to half of the desired dose are all recommended. The cost of interferon-alpha may also preclude its use in some patients.[20]
Anagrelide
Anagrelide hydrochloride (Agrylin), an oral quinazoline that inhibits cyclic nucleotide phosphodiesterase, is the only drug approved by the US Food and Drug Administration (FDA) for the treatment of thrombocythemia associated with essential thrombocythemia and polycythemia vera. The drug was originally noted for its antiaggregating effect on platelets, but tests on normal adults revealed an unanticipated, sudden, and progressively severe thrombocytopenia at doses lower than those needed for antiaggregation.[38,51] Because this has not been demonstrated in lower primates or other species, it is considered a species-specific action. A leading hypothesis is that a metabolic pathway present in most but not all humans, and in few if any other species, converts the molecule into an as yet unknown active metabolite.[51]
The mechanism of action of anagrelide has not been entirely elucidated, but it is known that the drug inhibits postmitotic megakaryocyte maturation and platelet budding.[52] It is, therefore, not a myelosuppressive agent but a drug that is specific for platelet reduction. A 10% drop in hematocrit has been observed in approximately 30% of patients, which may be due, at least in part, to hemodilution reflecting anagrelide-induced fluid retention.[32] However, it may be a biological effect resulting from the suppression of erythropoiesis. Anagrelide is indicated for thrombocythemia in myeloproliferative disorders to reduce an elevated platelet count and the risk of thrombosis, and to ameliorate associated symptoms including thrombohemorrhagic events.[1]
Clinical Trials: The pilot dose-ranging trial of anagrelide commenced in 1985, and the results were published in 1989.[53] Of the 20 patients enrolled, 17 had essential thrombocythemia, 2 had polycythemia vera, and 1 had CML. All but two patients with essential thrombocythemia responded with marked reductions in platelet counts.
These results led to formation of the Anagrelide Study Group and a subsequent trial in 577 patients, 424 of whom were evaluable. The median pretreatment platelet count was 990,000/µL. Patients were treated for a minimum of 4 weeks. Approximately 93% met the criteria for effective response, namely a 50% reduction in platelet count or a reduction in platelet count to 600,000/µL. The median time to achieving these goals was 11 days for patients with essential thrombocythemia and 15 days for patients with polycythemia vera. The median duration of first response was 28.6 months for essential thrombocythemia patients and 7.7 months for polycythemia vera patients, for an overall median of 16.7 months. Platelet counts in patients who withdrew from anagrelide therapy rose to pretreatment levels within 5 to 7 days.[54]
In 1997, Petitt summarized a subsequent analysis of 942 patients, including the Anagrelide Study Group mentioned above and additional patients treated on a compassionate basis. This group of patients included 58% with essential thrombocythemia, 12% with polycythemia vera, 19% with CML, and 11% with undifferentiated myeloproliferative disorders.[51] The mean pretreatment platelet count was 1,131,600/mL, and 86% had received prior treatment. Anagrelide therapy was indicated in young patients, those in whom other therapies had failed, and those who had been withdrawn from other therapies because of side effects. A complete response was defined as a 50% reduction in platelet count or reduction to 600,000/mL or more for at least 4 weeks. A partial response was defined as a 20% to 50% reduction from pretreatment levels for at least 4 weeks. All others were categorized as nonresponders. Among polycythemia vera patients, 66% responded completely, and 8% responded partially; among essential thrombocythemia patients, 73% responded completely and 9%, partially.[51]
TABLE 4
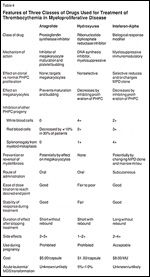
Features of Three Classes of Drugs Used for Treatment of Thrombocythemia in Myeloproliferative Disease
The efficacy of anagrelide has not yet been evaluated in randomized, double-blind head-to-head trials of interferon or hydroxyurea. Furthermore, additional evidence is needed before it can be concluded that, by controlling platelet counts, anagrelide is efficacious in reducing the frequency of thrombohemorrhagic events in patients with essential thrombocythemia and polycythemia vera.[9,29]
Safety: More is known of the drug’s safety profile. There is no evidence of oncogenicity or leukemogenicity. Hence, anagrelide therapy is safe for older patients whose symptoms are refractory to other treatments as well as for young patients, but in light of concerns that it may pass through the placenta, it is not advised for women trying to conceive.
Anagrelide has a broad but not especially profound side-effect profile. It is a vasodilator with positive inotropic activity that can reduce renal blood flow, increase fluid retention, and induce palpitations, forceful heartbeat, and tachycardia. Hence, it is contraindicated in patients with congestive heart failure or cardiac arrhythmias.[55] The most common side effect is headache, which usually responds to acetaminophen. Mild dizziness, nausea, bloating, and diarrhea usually abate in 2 to 4 weeks. Nevertheless, in various trials, 10% to 17% of subjects withdrew because of intolerable side effects. Most are clinically manageable. Anagrelide must be administered on a chronic basis, and no remarkable side effects have been associated with long-term use.
Because anagrelide is specific for platelet reduction, polycythemia vera patients taking it may require concurrent phlebotomy, hydroxyurea, or interferon-alpha to achieve control of erythrocythemia. In both polycythemia vera and essential thrombocythemia patients, combination therapy with anagrelide may reduce the undesirable side effects associated with hydroxyurea and interferon by prompting dose reductions.[13] Table 4 provides a comparative summary of the salient biological and clinical features of interferon-alpha, hydroxyurea, and anagrelide.[1]
References:
1. Gilbert HS: The role of anagrelide, hydroxyurea, andinterferon-a in treating thrombocythemia of myeloproliferative disease: A newapproach for the Millennium. International Society of Hematology program book,pp 141-143, 1999.
2. Gilbert HS: Diagnosis and treatment of polycythemia vera,agnogenic myeloid metaplasia, and essential thrombocythemia, in Wiernik PH,Canellos GP, Kyle RA, et al (eds): Neoplastic Diseases of the Blood, p 123. NewYork, Churchill Livingstone, 1991.
3. Sterkers Y, Preudhomme C, Lai JL, et al: Acute myeloidleukemia and myelodysplastic syndromes following essential thrombocythemiatreated with hydroxyurea. Blood 91(2):616-622, 1998.
4. Berk PD, Goldberg JD, Donovan PB, et al: Therapeuticrecommendations in polycythemia vera based on Polycythemia Vera Study Groupprotocols. Semin Hematol 23(2):132-143, 1986.
5. Berk PD, Wasserman LR, Fruchtman SM, et al: Treatment ofpolycythemia vera: A summary of clinical trials conducted by the PolycythemiaVera Study Group, in Wasserman LR, Berk PD, Berlin NI (eds): Polycythemia Veraand the Myeloproliferative Disorders, pp 102-113. Philadelphia, WB Saunders,1995.
6. Murphy S: Diagnostic criteria and prognosis in polycythemiavera and essential thrombocythemia. Semin Hematol 36(1 suppl 2):9-13, 1999.
7. Berlin NI: The diagnosis and classification of polycythemias.Semin Hematol 12:339-351, 1975.
8.Pearson TC, Messinezy M: The diagnostic criteria ofpolycythaemia rubra vera. Leuk Lymphoma 22 (suppl) 1:87-93, 1996.
9. Michiels JJ, Barbui T, Finazzi G, et al: Diagnosis andtreatment of polycythemia vera and possible future study designs of the PVSG.Leuk Lymphoma 36(3-4):239-253, 2000.
10. Thiele J, Kvasnicka HM, Diehl V, et al: Clinicopathologicaldiagnosis and differentiation of thrombocythemias in various myeloproliferativedisorders by histopathology, histochemistry, and immunostaining from bone marrowbiopsies. Leuk Lymphoma 33:207-218, 1999.
11. Tefferi A: Pathogenic mechanisms in chronicmyeloproliferative diseases: Polycythemia vera, essential thrombocythemia,agnogenic myeloid metaplasia, and chronic myelogenous leukemia. Semin Hematol36(1 suppl 2):3-8, 1999.
12. Gilbert HS: Familial myeloproliferative disease. BaillieresClin Haematol 11(4):849-858, 1998.
13. Gilbert HS: Polycythemia vera, in Rakel RE (ed): Conn’s2000: Latest Approved Methods of Treatment for the Practicing Physician, pp445-447. Philadelphia, WB Saunders, 2000.
14. Murphy S, Peterson P, Iland H, et al: Experience of thePolycythemia Study Group with essential thrombocythemia: A final report on thediagnosis criteria, survival, and leukemic transition by treatment. SeminHematol 34(1):29-39, 1997.
15. Wasserman LR, Berk PD, Berlin NI (eds): Polycythemia Veraand the Myeloproliferative Diseases. Philadelphia, WB Saunders, 1995.
16. Moliterno AR, Hankins WE, Spivak JL: Impaired expression ofthe thrombopoietin receptor by platelets from patients with polycythemia vera. NEngl J Med 338:572-580, 1998.
17. Horikawa Y, Matsumara I, Hashimoto K, et al: Markedlyreduced expression of platelet c-Mpl receptor in essential thrombocythemia.Blood 90:4031-4038, 1997.
18. Barbui T, Finazzi G: Treatment of polycythemia vera.Haematologica 83:1430149, 1998.
19. Pearson TC, Barbui T: The management of polycythemia vera.Hematology 2:55-64, 1997.
20. Elliott MA, Tefferi A: Interferon-alpha therapy inpolycythemia vera and essential thrombocythemia. Semin Thromb Hemost23(5):463-472, 1997.
21. Gruppo Italiano Studio Policithemia: Polycythemia vera: Thenatural history of 1213 patients followed for 20 years. Ann Intern Med123:656-664, 1995.
22. Michiels JJ: Erythromelalgia and vascular complications inpolycythemia vera. Semin Thromb Hemost 23:441-454, 1997.
23. Murphy S: Therapeutic dilemmas: Balancing the risks ofbleeding, thrombosis and leukemic transformation in myeloproliferative disorders(MPD). Semin Thromb Hemost 78(1):622-626, 1997.
24. Tefferi A, Elliott MA, Solberg LA, et al: New drugs inessential thrombocythemia and polycythemia vera. Blood 11:1-17, 1997.
25. Koudstaal PJ, Koudstaal A: Neurological and visual symptomsin essential thrombocythemia: Efficacy of low-dose aspirin. Semin Thromb Hemost23(4):365-370, 1997.
26. Iten PH, Winkelmann RK: Cutaneous manifestations in patientswith essential thrombocythemia. J Am Acad Dermatol 24:59-63, 1991.
27. Griesshammer M, Bangerter M, VanVliet HH, et al: Aspirin inessential thrombocythemia: Status quo and quo vadis. Semin Thromb Hemost23(4):371-377, 1997.
28. Mesa RA, Tefferi A, Jacobsen SJ, et al: The incidence andepidemiology of essential thrombocythemia and agnogenic myeloid metaplasia: AnOlmstead County study (abstract). Blood 90:347, 1997.
29. Barbui T, Finazzi G: Clinical parameters for determiningwhen and when not to treat essential thrombocythemia. Semin Hematol 36(1 suppl2):14-18, 1999.
30. Michiels JJ, Juvonen E: Proposal for revised diagnosticcriteria of essential thrombocythemia and polycythemia vera by the PolycythemiaVera Study Group. Semin Thromb Hemost 23(4):339-347, 1997.
31. Regev A, Stark P, Blickstein D, et al: Thromboticcomplications in essential thrombocythemia with relatively low platelet counts.Am J Hematol 56:168-172, 1997.
32. Gilliland DG, Silverstein MN, Anderson JE, et al:Myeloproliferative disorders and myelodysplastic syndromes, pp 166-167. AmericanSociety of Hematology Education Program Book, 1997.
33. Pearson TC, Weatherly-Mein G: Vascular occlusion episodesand venous hematocrit in primary proliferative polycythaemia. Lancet2:1219-1222, 1978.
34. Kannel WB, Gordan T, Wolf PA, et al: Hemoglobin and the riskof cerebral infarction: The Framingham study. Stroke 3:409-420, 1972.
35. Tohgi H, Yamanouche H, Murakami M, et al: The importance ofhematocrit as a factor in cerebral infarction. Stroke 9:369-374, 1978.
36. Thomas DJ, duBoulay GH, Marshall J, et al: Cerebral flow inpolycythemia. Lancet 2:161-163, 1977.
37. Wade JPH: Transport of oxygen to the brain of patients withelevated hematocrit values before and after venesection. Brain 106:513-523,1983.
38. Gilbert HS: Historical perspective on the treatment ofessential thrombocythemia and polycythemia vera. Semin Hematol 36(1 suppl2):19-22, 1999.
39. Najean ME, Rain JD: The very long-term evolution ofpolycythemia vera: An analysis of 318 patients initially treated by phlebotomyor 32P between 1969 and 1981. Semin Hematol 34:6-16, 1997.
40. Kaplan ME, Mack K, Goldberg JD, et al: Long-term managementof polycythemia vera with hydroxyurea: A progress report. Semin Hematol23:167-171, 1986.
41. Fruchtman SM, Mack K, Kaplan ME, et al: From efficacy tosafety: A Polycythemia Vera Study Group report on hydroxyurea in patients withpolycythemia vera. Semin Hematol 34:17-23, 1997.
42. Katarsky I, Sharon R: Management of polycythemia vera withhydroxyurea. Semin Hematol 34:24-28, 1997.
43. Weinfeld A, Swolin B, Westin J: Acute leukemia afterhydroxyurea treatment in polycythemia vera and allied disorders: Prospectivestudy of efficacy and leukemogenicity with therapeutic implications. Eur JHaematol 52:134, 1994.
44. Najean Y, Rain JD: Treatment of polycythemia vera: The useof hydroxyurea and pipobroman in 292 patients under the age of 65 years(abstract). Blood 90(9):3370-3377, 1997.
45. Gilbert HS: Remission of myeloid metaplasia induced byrecombinant alpha interferon (abstract) Clin Res 36:613a, 1988.
46. Gilbert HS: Long-term treatment of myeloproliferativedisease with interferon-alpha 2b. Cancer 83(6):1205-1213, 1998.
47. Silver RT: Interferon in the treatment of myeloproliferativediseases. Semin Hematol 27(3 suppl 4):6-14, 1990.
48. Silver RT: A new treatment for polycythemia vera:Recombinant interferon alfa (abstract). Blood 74(4):664-665, 1990.
49. Lengfelder E, Griesshammer M, Hehlmann R: Interferon-alphain the treatment of essential thrombocythemia. Leuk Lymphoma 22(suppl1):135-142, 1996.
50. Silver RT: Interferon-alpha 2b: A new treatment forpolycythemia vera. Ann Intern Med 119:1091-1092, 1993.
51. Petitt RM, Silverstein MN, Petrone ME: Anagrelide fortreatment of thrombocythemia in polycythemia and other myeloproliferativediseases. Semin Hematol 34(1):51-54, 1997.
52. Spencer CM, Brogden RN: Anagrelide: A review of itspharmacodynamic and pharmacokinetic properties and therapeutic potential in thetreatment of thrombocythemia. Drugs 47(5):809-822, 1994.
53. Silverstein MN, Petitt RM, Solberg LA Jr, et al: Anagrelide:A new drug for treating thrombocytosis. N Engl J Med 318:1292-1294, 1989.
54. Anagrelide Study Group: Anagrelide, a new therapy forthrombocythemic states: Experience in 577 patients. Am J Med 92:69-76, 1992.
55. Silverstein MN, Tefferi A: Treatment of essentialthrombocythemia with anagrelide. Semin Hematol 36(1 suppl 2):23-25, 1999.