Diffuse Malignant Mesothelioma of the Pleural Space and Its Management
Diffuse malignant pleural mesothelioma is a rare and aggressive malignancy of the pleura that is usually caused by exposure to asbestos. Between 2,000 and 3,000 new cases are expected to be diagnosed annually in the
ABSTRACT: Diffuse malignant pleural mesothelioma is a rare and aggressive malignancy of the pleura that is usually caused by exposure to asbestos. Between 2,000 and 3,000 new cases are expected to be diagnosed annually in the United States. Difficulties in diagnosis, staging, and treatment set this disease apart from other malignancies. The variable clinical presentation and problems in establishing a definite histopathologic diagnosis result in significant delays in treatment. Three histologic subtypes of the disease are described in this review: epithelial, sarcomatous, and mixed histologies. The Butchart, International Mesothelioma Interest Group, and Brigham staging systems are the most commonly used staging systems. The disease’s natural history involves aggressive local growth, invasion of vital mediastinal structures, and death within 4 to 12 months without treatment. Single-modality therapy of any kind has failed to substantially alter this natural history. Aggressive, multimodality regimens that include surgery, radiation, and chemotherapy have resulted in improved survival in properly selected patients. However, innovative therapies are still needed to prolong survival in patients with early and advanced disease. [ONCOLOGY 16:907-925, 2002]
Diffuse malignant pleural mesothelioma is a rare malignant tumor with an annual incidence in the United States of 2,000 to 3,000 cases.[1] Its natural history is characterized by local aggressiveness and invasion, which, left untreated, results in a median survival ranging between 4 and 12 months.[2] Although asbestos exposure remains the most important epidemiologic factor in the development of this disease, the importance of the simian virus 40 (SV40) has been recently recognized.[3]
Management of diffuse malignant pleural mesothelioma presents several challenges to physicians, from diagnosis and staging to treatment. The lack of randomized studies and the low incidence of this tumor are responsible for the absence of a consensus on how it should be treated.
Clinical Presentation
Mesothelioma occurs most commonly in males (3:1 male-to-female ratio), is unilateral in most patients (95%) with a slight right-sided preponderance, and develops as a result of exposure to asbestos after a median latency period of 32 years.[4] Although most patients are over age 55 at the time of presentation, cases have been reported in younger patients, including children. Such cases are due to causes other than asbestos exposure. The majority of patients present with symptoms related to pleural effusion, such as dyspnea, cough, and chest pain.[4] Other symptoms such as fatigue, weight loss, fever, and night sweats can also occur.
Initially, dyspnea develops as a result of pleural effusion, but as the tumor grows, it replaces the pleural space, and the lung becomes entrapped. Similarly, the chest pain that occurs due to the presence of pleural effusion is at first poorly localized and dull. As the tumor grows, however, intercostal nerves become entrapped, and the pain becomes localized. Unrelenting tumor growth results in mediastinal and abdominal invasion. Ascites, cachexia, chest and abdominal wall deformity, bowel obstruction, and cardiopulmonary compromise lead to the patient’s demise. Metastases in the brain, bone, and other organs rarely occur. However, such metastases are seen more frequently in patients who have received multimodality therapy.[5]
On physical examination, most mesothelioma patients with early disease manifest only signs of pleural effusion such as decreased breath sounds. With advanced disease, palpable chest or abdominal masses can be present. Abdominal masses are a particularly ominous sign because they indicate transdiaphragmatic involvement and unresectability.
Laboratory tests are not very informative, although thrombocytosis (> 400 × 109/L) is thought to be an indicator of poor prognosis.[6] Laboratory evaluation can reveal other nonspecific findings such as anemia of chronic disease, eosinophilia, and hypergammaglobulinemia.
Radiologic Work-up
Standard radiologic evaluation of mesothelioma patients should include a chest x-ray, computed tomography (CT) scan of the chest, and magnetic resonance imaging (MRI). Pleural effusions, plaques, and thickening can be seen on plain chest x-rays. However, resectability is best determined with CT and MRI scans, which can better assess the extent of invasion into the chest wall, mediastinum, and diaphragm.[7] Surgeons debate whether MRI or CT scan offers more information and better precision in determining resectability; we have found MRI to be more informative, but the use of both tests in conjunction is quite effective in determining resectability.
Fluorodeoxyglucose positron-emission tomography (PET) has been used with increasing frequency at our institution, mainly to assess distant occult disease. The increased sensitivity of this modality was documented in a recent study of 18 consecutive patients with diffuse malignant pleural mesothelioma. The PET scan detected the presence of distant disease in two patients despite negative CT scans and was more sensitive than CT in detecting mediastinal adenopathy. Another study correlated survival with intensity of uptake on PET scan, indicating an ability to quantify tumor burden.[8,9]
Two-dimensional echocardiography is another useful test with which to determine resectability. This technique is based on the assessment of pericardial involvement and cardiac function.
Pathologic Diagnosis
TABLE 1

Diagnostic Aids to Differentiate Malignant Pleural Mesothelioma From Adenocarcinoma
When patients present with unilateral pleural effusion, the physician needs to relieve the symptoms (by draining the fluid) and establish the cause of the effusion. The three most commonly used methods for achieving these goals are thoracentesis with cytology, closed pleural biopsy, and open pleural biopsy via video-assisted thoracic surgery.
Thoracentesis is usually employed in the initial evaluation and management of these effusions. The effusion is effectively drained, and the fluid can be sent for cytologic and chemical analysis. Mesothelioma-related effusions are typically clear yellow. Thoracentesis, however, establishes a diagnosis in only 30% to 35% of cases due to its inability to distinguish tumor cells from reactive mesothelial cells.[10] This low diagnostic yield has been improved with the assistance of histochemistry, immunohistochemistry, and electron microscopy.[11]
Closed pleural needle biopsy can be used in an attempt to obtain pleural tissue and improve the diagnostic yield.[12] However, for the most part, this is a blind technique with a high false-negative rate because the malignant mass may be missed and normal pleura biopsied instead. Video-assisted thoracic surgery is a minimally invasive technique that improves visualization, effectively drains all fluid by disrupting loculations, and provides an adequate number of tumor tissue samples for the various stains and electron microscopy.[13]
TABLE 2

International Mesothelioma Interest Group Staging System for Diffuse Malignant Pleural Mesothelioma
Regardless of the procedure used, tumor cell seeding occurs at the biopsy sites, and chest wall masses eventually develop. Therefore, it is important to strategically place thoracentesis or biopsy sites so that they can be resected at the time of future cytoreductive surgery.
The three histologic subtypes of diffuse malignant pleural mesothelioma that have been described are epithelial, sarcomatous, and mixed. Patients with the epithelial subtype have a much better prognosis than patients with tumors of the other cell types. For the pathologist, diffuse malignant pleural mesothelioma presents a challenge because of the difficulty in distinguishing it from adenocarcinoma and, occasionally, sarcoma. Table 1 lists the criteria used to differentiate diffuse malignant pleural mesothelioma from adenocarcinoma.[14-16]
Staging Systems
Numerous staging systems have been proposed for diffuse malignant pleural mesothelioma, but none have been widely accepted. A staging system should stratify survival and direct therapy. Butchart proposed the first staging system in 1976, based on 29 patients.[17] However, there was no correlation between stage and survival. Two tumor/node/metastasis (TNM) systems have been proposed: the International Union Against Cancer system[18] and the International Mesothelioma Interest Group system (Table 2).[19] These staging systems, however, still need to be validated.
FIGURE 1
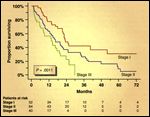
Surgery Survivors
The Brigham/Dana-Farber Cancer Institute staging system, proposed by Sugarbaker and colleagues in 1993[20] and later revised in 1998, is based on a series of 183 patients who underwent extrapleural pneumonectomy and adjuvant chemoradiation.[21] This system mainly defines resectability and nodal status. Positive resection margins or intrapleural nodes identify the disease as stage II, whereas positive extrapleural nodes or involvement of the diaphragmatic muscle or pericardium indicates stage III disease (Table 3 and Figure 1).[21] Median survival following extrapleural pneumonectomy and adjuvant chemoradiation in patients with stage I, II, and III disease is 25, 20, and 16 months, respectively.[21]
Treatment
Single-Modality Therapy
TABLE 3

The Revised Staging System for Malignant Pleural Mesothelioma
Single-modality therapy, regardless of type (surgery, chemotherapy, or radiotherapy), has failed to have a significant impact on survival. The two main surgical procedures used for cytoreduction of diffuse malignant pleural mesothelioma are pleurectomy/decortication and extrapleural pneumonectomy. To date, no randomized trial has compared these two surgical procedures, and the debate continues among surgeons as to which procedure is most appropriate.
Extrapleural pneumonectomy achieves the best cytoreduction. Operative mortality has been as high as 30% in old series with small numbers.[17] However, the largest modern series report mortality rates below 4%.[21]
Pleurectomy/decortication can be consistently performed with low morbidity and mortality (25% and 2%-5%, respectively) in most centers.[22] However, the limitations of the procedure include inadequate cytoreduction when the tumor has invaded the fissures, and the need to limit adjuvant therapy due to the continued presence of the lung. Proponents of pleurectomy/decortication point out that aggressive cytoreduction alone, such as that accomplished with extrapleural pneumonectomy, has resulted in a 5-year survival of only 10%.[17]
Similarly, radiotherapy alone has failed to improve survival. Its greatest utility is in preventing tumor development from thoracentesis and at sites of video-assisted thoracic surgery. A randomized trial conducted by Boutin et al revealed that irradiated sites of video-assisted thoracic surgery or thoracentesis had no tumor recurrence, compared to a 40% recurrence rate in nonirradiated sites.[23]
Various chemotherapeutic agents -including cyclophosphamide (Cytoxan, Neosar), methotrexate, doxorubicin, carboplatin (Paraplatin), paclitaxel, and cisplatin-have been used alone or in combination. Response rates have been disappointing (20%-30%), with methotrexate exhibiting the highest single-agent response rate of 37%.[24,25] Interestingly, the folate receptor has been found to be up-regulated in the majority of diffuse malignant pleural mesothelioma specimens regardless of histologic type, and new agents like the multitargeted antifolate pemetrexed (LY231514) might improve response rates.[26] Recent chemotherapeutic combinations such as cisplatin and gemcitabine (Gemzar) have achieved response rates as high as 48%.[27]
Multimodality Therapy
Multimodality regimens were developed to improve on the poor survival results seen with single-modality regimens. The goal of such protocols is to combine surgical cytoreduction with adjuvant radiation, chemotherapy, or both. Adjuvant therapies are thought to be more effective when maximum cytoreduction has been achieved; thus, chemotherapeutic agents do not need to penetrate the entire pleura, and a more focal area can be targeted by radiotherapy.
Both extrapleural pneumonectomy and pleurectomy/decortication have been incorporated into multimodality protocols. Extrapleural pneumonectomy achieves the best cytoreduction and allows higher doses of radiotherapy to be delivered in the ipsilateral hemithorax because the lung has been removed. Additionally, locoregional or intraoperative therapy can be delivered with less systemic absorption and toxicity. Although extrapleural pneumonectomy is associated with a higher morbidity and mortality than pleurectomy/decortication, multimodality regimens that include extrapleural pneumonectomy have produced longer survivals in patients with early-stage disease.[21]
In the multimodality setting, pleurectomy/decortication has been associated with mortality rates of 1.5% to 5.4% and median survivals of 9 to 17 months.[22] Intrapleural chemotherapy, immunotherapy, and radiotherapy have been administered either intraoperatively or in the adjuvant setting following cytoreduction with pleurectomy/decortication. In the study reported by Rusch et al, intrapleural cisplatin and adjuvant chemotherapy, administered after pleurectomy/decortication, resulted in a 3.7% mortality and 17-month median survival, with local recurrence in 80% of patients.[28] Another study reported the results of intrapleural seed implant with iodine-125 or iridium-192 following pleurectomy/decortication. The median survival in this study was 13 months.[22]
No randomized study has compared extrapleural pneumonectomy and pleurectomy/decortication as single modalities or as part of multimodality regimens. Although centers have reported low mortality rates following extrapleural pneumonectomy, patients undergoing the procedure represent a select group with adequate functional status.
• Brigham and Women’s Hospital Study-At Brigham and Women’s Hospital, 183 patients underwent extrapleural pneumonectomy and adjuvant chemoradiation from 1980 to 1997 (since 1994, carboplatin at an area under the concentration-time curve [AUC] of 6 and paclitaxel at 200 mg/m2; the radiation dose was 40 Gy with a boost up to 54 Gy). Mortality among these patients was 3.8%, and the median survival, 17 months. Although these results are equivalent to those achieved with pleurectomy/decortication and adjuvant chemoradiation, a subset of patients with stage I disease (revised Brigham/Dana-Farber Cancer Institute staging system) and an epithelial cell type had a median survival of 51 months. This remains the highest median survival reported for a subset of mesothelioma patients.[21]
Patients in this protocol were selected based on the absence of significant comorbid disease (eg, cardiovascular, renal, hepatic), a Karnofsky performance score of 70 or greater, and sufficient physiologic reserve to tolerate an extrapleural pneumonectomy (as suggested by predicted postoperative forced expiratory volume in 1 second > 1 L, PCO2 < 45 mm Hg, PO2 > 65 mm Hg, and ejection fraction > 45%).[20,21]
Appropriate candidates for extrapleural pneumonectomy and adjuvant therapy include those with an epithelial histology, early-stage disease, and good functional status. While multimodality treatment that incorporates a pneumonectomy is the treatment of choice at Brigham and Women’s Hospital, patients who are not appropriate candidates for the procedure are offered pleurectomy/decortication and adjuvant chemoradiation.
Innovative Approaches to Treatment
Investigators have identified a subset of patients who clearly benefit from multimodality therapy and extrapleural pneumonectomy. However, patients with disease more advanced than stage I, those who cannot tolerate the procedure, and those with mixed or sarcomatous histologic subtypes have not achieved a significant increase in survival. Other approaches or therapies are needed for these patients.[20,21]
• Photodynamic Therapy-Photodynamic therapy has been used following pleurectomy/decortication or extrapleural pneumonectomy with variable success. Pass and colleagues conducted a randomized phase III trial in 48 patients who underwent cytoreductive surgery, with or without intraoperative photodynamic therapy followed by adjuvant cisplatin, interferon alfa-2b (Intron A), and tamoxifen. No differences in median survival (14.4 vs 14.1 mo) were reported.[29] Most patients in the study had stage III disease.
Moskal and colleagues reported their experience in 40 patients who underwent intracavitary photodynamic therapy after cytoreductive surgery. Patients with early disease (stages I/II) had a 36-month median survival; those with advanced disease (stages III/IV) had a median survival of only 10 months.
Thus, photodynamic therapy has demonstrated a benefit in early-stage disease, but no improvement in survival has been seen in patients with advanced disease.[30] The introduction of newer photosensitizing agents and improvements in the depth of penetration and method of light delivery may produce better results.
• Immunotherapy-Immunotherapy involves the use of cytokines to enhance the patient’s immune system and antitumor response. Mesotheliomas can secrete substances such as transforming growth factor (TGF)-beta, insulin-like growth factor (IGF)-1, interleukin (IL)-6, and platelet-derived growth factors A and B. TGF-beta is known to inhibit the proliferation of cells with antitumor activity such as natural killer cells.[31,32]
All three types of interferon have been tested in mesothelioma patients, with interferon-gamma (Actimmune) showing the most promise. Boutin et al noted an overall response rate of 19% in 89 patients with stage I/II (Butchart) diffuse malignant pleural mesothelioma who received intrapleural interferon-gamma. However, the response rate was 61% among patients with disease localized to the parietal and diaphragmatic pleura, with eight patients achieving a thoracoscopically proven complete response.[33]
IL-2 has also been administered as a single agent intrapleurally. Phase I and II studies have shown a response rate of 37%. Additionally, a randomized study that compared intracavitary interferon-alpha, interferon-beta, and IL-2 for the treatment of malignant effusions including mesothelioma-related effusion showed a superior response rate for IL-2.[34-36]
• Gene Therapy-Gene therapy, in general, involves the use of viruses to transfer genetic material into either immune cells or tumor cells, to alter biological activity and prolong patient survival. Adenoviruses have been used most commonly to this end. Kaiser et al used adenoviral vectors to transfer the herpes simplex virus thymidine kinase gene (HSV-tk) into mesothelioma tumor cells intrapleurally. Although HSV-tk gene expression was noted in 12 of 20 patients, no objective responses or effect on survival was reported.[37]
• Intracavitary Heated Chemotherapy-The rationale for intracavitary (ie, intrapleural) heated chemotherapy is to achieve much higher local drug concentrations with lower systemic toxicity than is possible with systemic administration. Hyperthermia has been known to improve response to chemotherapeutic agents.[38] Although cisplatin has been the most commonly used chemotherapeutic agent for intrapleural administration after extrapleural pneumonectomy or pleurectomy/decortication, no dose-escalating phase I study of extrapleural pneumonectomy has been conducted.
A phase I dose-escalating trial of intraoperative, intracavitary, heated chemotherapy after extrapleural pneumonectomy for diffuse malignant pleural mesothelioma was recently completed at Brigham and Women’s Hospital, with a similar trial near completion in patients who underwent pleurectomy/decortication. These two studies should determine the safety and efficacy of this approach and establish the appropriate dose of cisplatin in this setting.[39]
Summary
Diffuse malignant pleural mesothelioma remains a difficult tumor to diagnose, stage, and treat. Referral to a tertiary center that is experienced in all aspects of this disease is crucial, because delays in diagnosis or treatment result in tumor progression as well as decreased chances for effective cytoreduction and participation in multimodality regimens. Although multimodality regimens have not resulted in markedly improved survival across all stages and histologic types of the disease, they have benefited a select subset of patients. Further investigations are needed to improve survival in patients with advanced-stage disease.
Acknowledgment:The authors wish to thank Mary S. Visciano for editorial assistance.
References:
1. Connelly RR, Spirtas R, Myers MH, et al: Demographic patterns formesothelioma in the United States. J Natl Cancer Inst 78:1053, 1987.
2. Antman K, Shemin R, Ryan L, et al: Malignant mesothelioma: Prognosticvariables in a registry of 180 patients, the Dana-Farber Cancer Institute andBrigham and Women’s Hospital experience over two decades, 1965-1985. J ClinOncol 6:47, 1988.
3. Pass HI, Donington JS, Wu P, et al: Human mesotheliomas contain the simianvirus-40 regulatory region and large tumor antigen DNA sequences. J ThoracCardiovasc Surg 116(5):854-859, 1998.
4. Sugarbaker DJ, Garcia JP, Richards WG, et al: Extrapleural pneumonectomyin the multimodality therapy of malignant pleural mesothelioma. Results in 120consecutive patients. Ann Surg 224(3):288-294, 1996.
5. Baldini EH, Recht A, Strauss GM, et al: Patterns of failure aftertrimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg63(2):334-338, 1997.
6. Herndon JE, Green MR, Chahinian AP, et al: Factors predictive of survivalamong 337 patients with mesothelioma treated between 1984 and 1994 by the Cancerand Leukemia Group B. Chest 113(3):723-731, 1998.
7. Patz EF Jr, Shaffer K, Piwnica-Worms DR, et al: Malignant pleuralmesothelioma: Value of CT and MR imaging in predicting resectability. Am JRoentgen 159:961, 1992.
8. Schneider DB, Clary-Macy C, Challa S, et al: Positron-emission tomographywith f18-fluorodeoxyglucose in the staging and preoperative evaluation ofmalignant pleural mesothelioma. J Thorac Cardiovasc Surg 120(1):128-133, 2000.
9. Benard F, Sterman D, Smith RJ, et al: Prognostic value of FDG PET imagingin malignant pleural mesothelioma. J Nucl Med 40(8):1241-1245, 1999.
10. Renshaw AA, Dean BR, Antman KH, et al: The role of cytologic evaluationof pleural fluid in the diagnosis of malignant mesothelioma. Chest 111:106-109,1997.
11. Dejmek A: Methods to improve the diagnostic accuracy of malignantmesothelioma. Respir Med 90:191-199, 1997.
12. Beauchamp HD, Kundra NK, Aranson R, et al: The role of closed pleuralneedle biopsy in the diagnosis of malignant mesothelioma of the pleura. Chest102:1110-1112, 1992.
13. Boutin C, Rey F: Thoracoscopy in pleural malignant mesothelioma: Aprospective study of 188 consecutive patients. Part 1: Diagnosis. Cancer72:389-393, 1993.
14. Sugarbaker DJ, Norberto JJ, Bueno R: Current therapy for mesothelioma.Cancer Control 4:326-334, 1997.
15. Sugarbaker DJ, Reed MF, Swanson SJ: Mesothelioma in Sabiston DC Jr (ed):Textbook of Surgery: The Biological Basis of Modern Surgical Practice, 15th ed.Philadelphia, WB Saunders, 1996.
16. Johansson L, Linden CJ: Aspects of histopathologic subtype as aprognostic factor in 85 pleural mesotheliomas. Chest 109:109-114, 1996.
17. Butchart EG, Ashcroft T, Barnsley WC, et al: Pleuropneumonectomy in themanagement of diffuse malignant mesothelioma of the pleura. Experience with 29patients. Thorax 31:15-24, 1976.
18. Rusch WV, Ginsberg RJ: New concepts in the staging of mesotheliomas, inDeslauriers J, Lacquet LK (eds): Thoracic Surgery: Surgical Management ofPleural Diseases, p 3340. St. Louis, Mosby, 1990.
19. Rusch VW: The International Mesothelioma Interest Group. A proposed newinternational TNM staging system for malignant pleural mesothelioma. Chest108:1122-1128, 1995.
20. Sugarbaker DJ, Strauss GM, Lynch TJ, et al: Node status has prognosticsignificance in the multimodality therapy of diffuse, malignant mesothelioma. JClin Oncol 11:1172, 1993.
21. Sugarbaker DJ, Flores RM, Jaklitsch MT, et al: Resection margins,extrapleural nodal status, and cell type determine postoperative long-termsurvival in trimodality therapy of malignant pleural mesothelioma: Results in183 patients. J Thorac Cardiovasc Surg 117:54-65 (incl discussion), 1999.
22. Rusch VW: Pleurectomy/decortication in the setting of multimodalitytreatment for diffuse malignant pleural mesothelioma. Semin Thorac CardiovascSurg 9:367-372, 1997.
23. Boutin C, Rey F, Viallat JR: Prevention of malignant seeding afterinvasive diagnostic procedures in patients with pleural mesothelioma. Arandomized trial of local radiotherapy. Chest 108:754-758, 1995.
24. Ryan CW, Herndon J, Vogelzang NJ: A review of chemotherapy trials formalignant mesothelioma. Chest 113(suppl):66S-73S, 1998.
25. Taub RN, Antman KH: Chemotherapy for malignant mesothelioma. Semin ThoracCardiovasc Surg 9:361-366, 1997.
26. Bueno R, Appasani K, Mercer H, et al: The alpha folate receptor is highlyactivated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg121(2):225-233, 2000.
27. Byrne MJ, Davidson JA, Musk AW, et al: Cisplatin and gemcitabinetreatment for malignant mesothelioma: A phase II study. J Clin Oncol17(1):25-30, 1999.
28. Rusch V, Saltz L, Venkatraman E, et al: A phase II trial ofpleurectomy/decortication followed by intrapleural and systemic chemotherapy formalignant pleural mesothelioma. J Clin Oncol 12:1156, 1994.
29. Pass HI, Temeck BK, Kranda K, et al: Phase III randomized trial ofsurgery with or without intraoperative photodynamic therapy and postoperativeimmunotherapy for malignant pleural mesothelioma. Ann Surg Oncol 48:628-633,1997.
30. Moskal TL, Dougherty TJ, Urschel JD, et al: Operation and photodynamictherapy for pleural mesothelioma: 6-year follow-up. Ann Thorac Surg66:1128-1133, 1998.
31. Maeda J, Ueki N, Ohkawa T, et al: Transforming growth factor-beta 1(TGF-beta 1)- and beta 2-like activities in malignant pleural effusions causedby malignant mesothelioma or primary lung cancer. Clin Exp Immunol 98:319-322,1994.
32. Jagirdar J, Lee TC, Reibman J, et al: Immunohistochemical localization oftransforming growth factor beta isoforms in asbestos-related diseases. EnvironHealth Perspect 105(suppl 5):1197-1203, 1997.
33. Boutin C, Nussbaum E, Monnet I, et al: Intrapleural treatment withrecombinant gamma-interferon in early-stage malignant pleural mesothelioma.Cancer 74:2460-2467, 1994.
34. Goey SH, Eggermont AM, Punt CJ, et al: Intrapleural administration ofinterleukin 2 in pleural mesothelioma: A phase I-II study. Br J Cancer72(5):1283-1288, 1995.
35. Astoul P, Picat-Joossen D, Viallat JR, et al: Intrapleural administrationof interleukin-2 for the treatment of patients with malignant pleuralmesothelioma: A phase II study. Cancer 83:2099-2104, 1998.
36. Lissoni P, Barni S, Tancini G, et al: Intracavitary therapy of neoplasticeffusions with cytokines: Comparison among interferon alpha, beta andinterleukin-2. Support Care Cancer 3:78-80, 1995.
37. Sterman DH, Kaiser LR, Albelda SM: Gene therapy for malignant pleuralmesothelioma. Hematol Oncol Clin North Am 12:553-568, 1998.
38. Ratto GB, Civalleri D, Esposito M, et al: Pleural space perfusion withcisplatin in the multimodality treatment of malignant mesothelioma: Afeasibility and pharmacokinetic study. J Thorac Cardiovasc Surg 117:759-765,1999.
39. Grondin SC, Sugarbaker DJ: Malignant mesothelioma of the pleural space.Oncology 13:919-926, 1999.