Strategies for the Optimal Management of Dyspnea in Cancer Patients With Advanced Illness
In this article, Dr. Mona Patel discusses how to best manage dyspnea in cancer patients, with both pharmacologic and nonpharmacologic approaches.
Oncology (Williston Park). 32(12):583-5, 590.

Mona S. Patel, DO

Table 1. Causes of Dyspnea in Cancer Patients
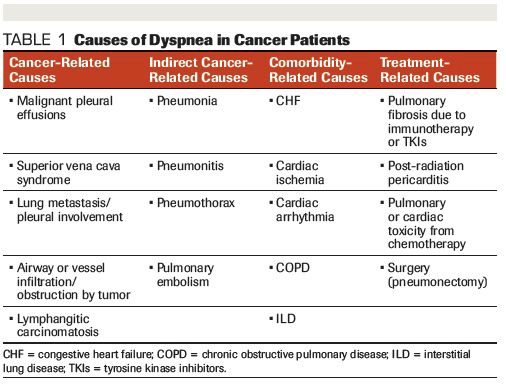
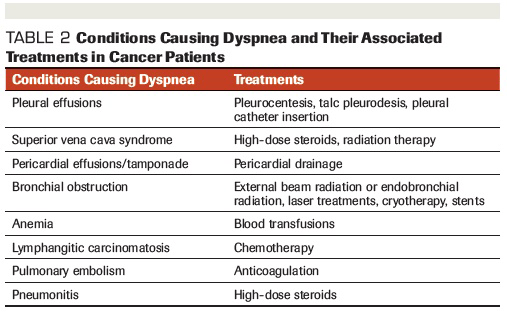
Table 2. Conditions Causing Dyspnea and Their Associated Treatments in Cancer Patients
Dyspnea in cancer patients can lead to significant deleterious effects. There are multiple conditions that can cause dyspnea. It is important to determine which of these causes are potentially reversible and treatable, so that they can be promptly addressed. Both pharmacologic and nonpharmacologic approaches should be used to treat this difficult condition. As patients advance in their illness, palliative treatments can be considered, such as low-dose opioids, oxygen therapy, and treatments directed at anxiety relief. Physicians should also discuss goals of care with their patients.
Introduction
Dyspnea is a common condition in patients with advanced cancer, with studies reporting a prevalence of 15% to 55% at initial diagnosis, and 40% to 90% during terminal stages.[1,2] The American Thoracic Society defines dyspnea as a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. It is influenced by psychological, physical, environmental, and social factors.[3,4]
Dyspnea can affect a patient’s quality of life by reducing activity level, functional capacity, and performance status, causing distress to both the patient and his or her family. This can lead to depression, anxiety, social isolation, and fear. Anxiety is a prominent symptom associated with dyspnea, and one tends to perpetuate the other.
Dyspnea has been shown to cause suffering, not only for cancer patients, but for their caregivers as well. Dyspnea in lung cancer patients is the most frequent cause for admission to the hospital. The prevalence of dyspnea also increases as patients near death.[5-7]
Clinicians should be aware that, like pain, dyspnea is often reported by patients who may not have tachypnea or appear to be dyspneic. It is a subjective condition that is reported by the patient. In a study of hospice patients, tachypnea did not correlate with patient-reported dyspnea. Of the patients studied, 77% reported dyspnea, but only 39% were recorded as having symptoms.[8]
Pathophysiology
The pathophysiology of dyspnea is complex and multifactorial. It is poorly understood, given the many factors that play a role in the experience. These include mechanical, chemoreceptor, and environmental factors. Often dyspnea occurs when a patient experiences hypoxia, metabolic acidosis, and hypercapnia, or when a patient engages in exercise stimulating the respiratory drive.
One mechanism that can drive dyspnea is when peripheral chemoreceptors in the carotid bodies and aorta respond to hypoxemia. Another mechanism that can occur is when central chemoreceptors in the brain respond to increases in carbon dioxide. A third occurs when mechanoreceptors in the lungs, chest wall, and airways respond to stretch, resulting in dyspnea. An example of this is when patients have airflow obstruction and there is an increase in mechanical resistance. Alternatively, juxtacapillary receptors at the junction of the alveoli and capillaries respond to alveolar fluid or microemboli, resulting in dyspnea.[9,10]
Etiology and Assessment
Dyspnea can be caused by a number of reversible and irreversible factors. In cancer patients, it can be caused by factors related to the cancer itself, a patient’s comorbid conditions, or by the treatment given for the cancer.
It is important to determine the underlying cause of the dyspnea to optimize treatment. In most instances, however, the etiology of dyspnea in advanced illness is likely to be multifactorial. Table 1 lists some of the common causes of dyspnea in cancer patients.
First and foremost, a thorough history and physical examination should be conducted to determine the underlying cause or causes of dyspnea. Basic testing such as chest x-ray, electrocardiogram, complete blood count, electrolyte testing, and pulse oximetry should be the next step(s). However, it is important to note that no specific cause of dyspnea is identified in approximately 25% of patients.[10]
To assess the degree of dyspnea that a patient is experiencing, a number of scales can be used. The most common ones use the patient’s self-report. The easiest-to-use dyspnea scales are the verbal, numerical, and visual analog scales. The Numerical Rating Scale (NRS) rates breathlessness from 0–10, with 0 = no breathlessness and 10 = worst breathlessness possible. The Visual Analog Scale is usually utilized for the daily assessment of intensity of dyspnea. Another scale that uses numbers with corresponding text is the Borg Scale, which has been used in advanced cancer patients. The Cancer Dyspnea Scale (CDS) evaluates physical dyspnea, as well as 12 items that highlight physical factors, psychological factors, and factors related to the patient’s sense of discomfort. Finally, for patients who are unable to self-report, there is the Respiratory Distress Observation Scale. This uses a number of observation parameters to assess for dyspnea, including heart rate, respiratory rate, accessory muscle use, paradoxical breathing pattern, restlessness, grunting at end-expiration, nasal flaring, and a fearful facial display.[11]
Management
The cornerstone of treatment for dyspnea is to determine and treat its underlying cause. Reversible causes should be addressed and managed promptly (Table 2).
Patients with cancer who have comorbidities contributing to their dyspnea should undergo treatments directed at the comorbid condition. Many cancer patients may have chronic obstructive pulmonary disease (COPD) or asthma that causes dyspnea and should be managed with bronchodilators, corticosteroids, and oxygen. Antibiotics may be appropriate if there is suspicion of pneumonia or bronchitis. Patients with congestive heart failure as a comorbidity should be treated with oxygen and diuretics.
Patients with cancer may have malignant pleural or pericardial effusions, which can be treated with pleurocentesis or pericardiocentesis, respectively. Another complication of malignancy is superior vena cava syndrome. These patients typically respond well to high-dose steroids and radiation therapy. Dyspnea can also present in patients with lymphangitic carcinomatosis, which can improve with corticosteroids and chemotherapy.[12] Patients who have anemia can benefit from blood transfusions to relieve dyspnea. For patients with obstruction of a bronchus, radiation and bronchial stents may be beneficial, along with laser or cryotherapy as alternative options.
Clinicians should also have a high index of suspicion for dyspnea caused by pulmonary toxicity and pneumonitis from treatment with immunotherapy agents, as well as interstitial lung disease (ILD) caused by tyrosine kinase inhibitors (TKIs). While ILD is a rare complication of TKI use, with a variable incidence rate of 0.2% to 10.9%, it can cause significant clinical symptoms and at times can be fatal.[13] Pulmonary toxicity from these agents often presents with variable clinical and radiographic changes, and should be treated promptly by stopping the offending drug and with administration of corticosteroids for immunosuppression. Outcomes are generally favorable, but it’s important to note that toxicity relapses can occur, even in the absence of rechallenge with the agent.[13]
Patients with malignancy are at high risk for developing thrombosis and pulmonary emboli, for which anticoagulation is indicated. Other causes of dyspnea, including ascites or pleural effusions, can be treated with fluid removal.
Mehmet Sitki Copur, MD, FACP
Dyspnea: A Holistic Approach
Dyspnea, the highly threatening experience of breathlessness, can turn simple daily activities for a cancer patient into a major challenge. What is striking and difficult about dyspnea is that by definition it is a subjective feeling. In 25% of the cases, no specific cause can be identified. Yet, it has a very significant impact on quality of life, and not only for patients; family and friends endure great emotional suffering when their loved one is facing loss of independence and increasing physical distress. Dyspnea often leads to severe anxiety, which often causes dyspnea. The psychosocial dimensions and environmental conditions play a major role in this clinical condition.
Dr. Patel nicely reviews the conditions that cause dyspnea in cancer patients and recommends effective treatment strategies. The real challenge, however, is in the management of patients in whom no specific cause of dyspnea can be established. A cancer patient’s self-report of dyspnea may encompass physical, as well as psychological, social, and spiritual, domains. Thus, the term “total dyspnea” has been proposed to be similar to “total pain” to capture the complexity of this condition. The total dyspnea model tries to comprehensively define the suffering of the patient experiencing dyspnea. It describes the patient’s experience in a broader manner to incorporate the wide spectrum of new treatment interventions and approaches, spanning from pharmacological and mechanical strategies, to behavioral strategies, for symptom management and resolution. Utilization of a multidisciplinary approach focusing on the patient’s psychological, social, and spiritual needs, as well as on the physical symptoms, are highlighted in this model.
As pointed out by Dr. Patel, while the body of literature continues to grow in this field, the need persists for further research regarding management strategies for dyspnea. Future research addressing the role of modulating factors and the mechanisms involved in dyspnea processing, particularly on the effects of attention, self-awareness, and distraction, are desperately needed. Integrating behavioral measures and carefully designed neuroimaging protocols to define the neural pathways of the respiratory network in perception and modulation of dyspnea would be invaluable.
Last but not least, Dr. Patel brings awareness to one more very important aspect of cancer care that is currently being underutilized by oncology providers; the integration of palliative care into standard oncology care. Patients with advanced cancer should receive dedicated palliative care services early in their disease course concurrent with active treatment. One of the essential components of palliative care is symptom, distress, and functional status management, which includes the management of dyspnea.
Dr. Copur is a Medical Oncologist/Hematologist at Morrison Cancer Center, Mary Lanning Healthcare in Hastings, Nebraska, and is a Professor at the University of Nebraska Medical Center in Omaha, Nebraska.
Dyspnea-associated anxiety can usually be managed with pharmacologic treatments, such as selective serotonin reuptake inhibitors or with benzodiazepines during more advanced stages of illness, as well as nonpharmacologic measures, such as breathing exercises, relaxation techniques, and psychosocial support.
As patients advance in their disease process, palliative therapies may be necessary for symptom relief. Both pharmacologic and nonpharmacologic treatments can be administered to assist patients with symptom management of dyspnea to improve quality of life. When considering what therapies are best, it is important to discuss the goals of care with the patient and family.
Simple measures such as using a fan to blow air on the face or having the patient positioned in front of an open window have been described as helpful for symptom management of dyspnea in cancer patients.[14-17] Other nonpharmacologic strategies include pacing activities, avoiding exertion, and keeping calm to reduce anxiety. The benefits of nonpharmacologic interventions include the fact that they are inexpensive, convenient, have no adverse effects, and are available in many different settings.
Oxygen use for relief of dyspnea has been shown to be beneficial, but only for patients with hypoxemia. It is postulated that ambient air may provide temporary symptomatic relief of dyspnea via a placebo effect caused by the passage of increased airflow along the nasal passageways, which decreases anxiety. According to a multicenter, double-blind, randomized controlled trial, supplemental oxygen was shown to provide no additional benefit for refractory dyspnea in patients with partial pressure of oxygen ≥ 55 mm Hg compared with room air via nasal cannula.[18] Similarly, in a 2012 systematic review and meta-analysis of patients with lung cancer and lung metastasis, researchers found no advantage of supplemental oxygen compared with room air for alleviating dyspnea in cancer patients that were not hypoxemic.[19]
Opioids have been used for symptomatic relief of dyspnea, especially for patients who are advancing in their illness.[20-23] A systematic review and meta-analysis demonstrated a beneficial effect of opioid treatment for dyspnea in patients with lung cancer and lung metastasis.[19] Other studies have also found that low-dose oral morphine may be helpful for symptom management of breathlessness in patients with advanced respiratory and cardiac conditions.[24] In terms of the route of delivery of opioids, the evidence favors the oral and parenteral routes.[24] A Cochrane review in 2001 found no benefit for nebulized opioids over other routes of delivery for relief of dyspnea in advanced cancer patients. However, the evidence and quality of these studies were limited.[25] Of the opioids, morphine has been the most widely studied for the treatment of dyspnea. The other opioids are postulated to have comparable effects on dyspnea given their similar mechanisms of action. However, proof of efficacy of fentanyl in particular is lacking and requires further investigation in randomized controlled trials.[26]
Clinicians often cite concerns for respiratory depression when using opioids, hence their reluctance to use them in the advanced chronically ill patient with dyspnea. However, a meta-analysis showed no evidence of clinically significant respiratory depression in those with chronic breathlessness treated with regular low-dose opioids.[27]
As previously mentioned, anxiety is a common symptom that patients with dyspnea experience. Benzodiazepines may provide relief from anxiety, thereby providing relief from dyspnea as well. However, a Cochrane review did not show evidence for or against the use of benzodiazepines for the symptomatic treatment of dyspnea.[28] There is also concern regarding the drowsiness that benzodiazepines can cause, and they are typically reserved for patients during terminal stages.
Patients with advanced cancer may also experience dyspnea caused by fatigue and respiratory muscle wasting. Typically, breathing exercises and relaxation techniques have been used for symptom relief. Acupuncture has also been considered to assist with dyspnea relief; however, the evidence does not support or refute its effectiveness.[29]
Another modality that is used in patients who have respiratory distress is noninvasive positive pressure ventilation (NPPV). NPPV can be helpful to patients who have respiratory muscle fatigue, either from neuromuscular disorders or COPD. NPPV can be continuous or intermittent and is administered via facial or nasal masks. The majority of patients studied with NPPV are those with COPD. However, patients with cancer can have respiratory muscle weakness causing dyspnea and could potentially also benefit from NPPV.[9]
Clinicians should discuss the goals of care and preferences regarding advance directives with patients who have advanced cancer. These conversations should also include preferences on mechanical ventilation, hospitalization, code status, and palliative measures.
Conclusion
Dyspnea is a common and distressing condition in cancer patients, and is often difficult to treat. While the body of literature continues to grow in this field, the need persists for further studies and research regarding management strategies for dyspnea.
Financial Disclosure: Dr. Patel has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Edmonds P, Karlsen S, Khan S, Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med. 2001;15:287-95.
2. Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89:234-6.
3. American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159:321-40.
4. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435-52.
5. Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care. 2003;1:337-44.
6. Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5:90-100.
7. Currow DC, Smith J, Davidson PM, et al. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39:680-90.
8. Thomas JR, von Gunten CF. Management of dyspnea. J Support Oncol. 2003;1:23-32; discussion 32-4.
9. Pisani L, Hill NS, Pacilli AMG, et al. Management of dyspnea in the terminally ill. Chest. 2018;154:925-34.
10. Abrahm JL. A physician’s guide to pain and symptom management in cancer patients. 3rd ed. Baltimore, MD: Johns Hopkins University Press. p 461.
11. Bausewein C, Farquhar M, Booth S, et al. Measurement of breathlessness in advanced disease: a systematic review. Respir Med. 2007;101:399-410.
12. Hui D, Kilgore K, Frisbee-Hume S, et al. Dexamethasone for dyspnea in cancer patients: a pilot double-blind, randomized, controlled trial. J Pain Symptom Manage. 2016;52:8-16.
13. Peerzada MM, Spiro TP, Daw HA. Pulmonary toxicities of tyrosine kinase inhibitors. Clin Adv Hematol Oncol. 2011;9:824-36.
14. Wong SL, Leong SM, Chan CM, et al. The effect of using an electric fan on dyspnea in Chinese patients with terminal cancer. Am J Hosp Palliat Care. 2017;34:42-6.
15. Kako J, Morita T, Yamaguchi T, et al. Fan therapy is effective in relieving dyspnea in patients with terminally ill cancer: a parallel-arm, randomized controlled trial. J Pain Symptom Manage. 2018;56:493-500.
16. Galbraith S, Fagan P, Perkins P, et al. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage. 2010;39:831-8.
17. Puspawati NLPD, Sitorus R, Herawati T. Hand-held fan airflow stimulation relieves dyspnea in lung cancer patients. Asia Pac J Oncol Nurs. 2017;4:162-7.
18. Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnea: a double-blind, randomised controlled trial. Lancet. 2010;376:784-93.
19. Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51:996-1008.
20. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: a national prospective study. BMJ. 2014;348:g445.
21. Currow DC, McDonald C, Oaten S, et al. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage. 2011;42:388-99.
22. Ekström M, Nilsson F, Abernethy AA, Currow DC. Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease: a systematic review. Ann Am Thorac Soc. 2015;12:1079-92.
23. Viola R, Kiteley C, Lloyd NS, et al. The management of dyspnea in cancer patients: a systematic review. Support Care Cancer. 2008;16:329-37.
24. Abernethy AP, Currow DC, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327:523-8.
25. Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev. 2001.
26. Simon ST, Köskeroglu P, Gaertner J, Voltz R. Fentanyl for the relief of refractory breathlessness: a systematic review. J Pain Symptom Manage. 2013;46:874-86.
27. Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, et al. Respiratory adverse effects of opioids for breathlessness: a systematic review and meta-analysis. Eur Respir J. 2017;50:1701153.
28. Simon ST, Higginson IJ, Booth S, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and nonâmalignant diseases in adults. Cochrane Database Syst Rev. 2016;10:CD007354.
29. Wu X, Chung VC, Hui EP, et al. Effectiveness of acupuncture and related therapies for palliative care of cancer: overview of systematic reviews. Sci Rep. 2015;5:16776.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.