Efficacy Controls and Long-Term Follow-Up of Patients Treated With Rituximab for Relapsed or Refractory, Low-Grade or Follicular Non-Hodgkin’s Lymphoma
Rituximab (Rituxan), a chimeric monoclonal antibody (MoAb), binds with high affinity to the CD20 antigen found on malignant and normal B-cells, but not on other normal tissues. CD20 is an attractive target because of the accessibility and sensitivity of malignant B-cells to lysis via immune effector mechanisms. This MoAb mediates complement-dependent cell lysis and antibody-dependent cellular cytotoxicity. Also, it has been shown to sensitize chemoresistant human lymphoma cell lines and to induce apoptosis in vitro.
Rituximab (Rituxan), a chimeric monoclonal antibody (MoAb), binds with high affinity to the CD20 antigen found on malignant and normal B-cells, but not on other normal tissues. CD20 is an attractive target because of the accessibility and sensitivity of malignant B-cells to lysis via immune effector mechanisms. This MoAb mediates complement-dependent cell lysis and antibody-dependent cellular cytotoxicity. Also, it has been shown to sensitize chemoresistant human lymphoma cell lines and to induce apoptosis in vitro.
In a single-agent, pivotal trial in 166 patients with refractory, low-grade or follicular non-Hodgkins lymphoma (NHL) (International Working Formulation [IWF] types A, B, C, D) treated with rituximab at 375 mg/m² weekly for four infusions, the overall response rate (ORR) was 48% (6% complete and 42% partial responses). Responses (computed tomographic [CT] scans) were confirmed in a blinded audit by independent lymphoma experts (LEXCOR panel) using rigorous response criteria. Median time to progression for responders was 13.2 months, and median duration of response was 11.6 months.
Of 80 responders, 20 remain in ongoing remission at 19.3+ to 36.7+ months. The ORR did not vary significantly with the number of prior courses of chemotherapy: one, two, or three courses (P = .19) but decreased, as expected, with the number of relapses: one, two, or three (P = .04), ranging from 57% to 38%. Refractory patients with zero relapses (never responded, never relapsed) had an ORR of 29% (6/21).
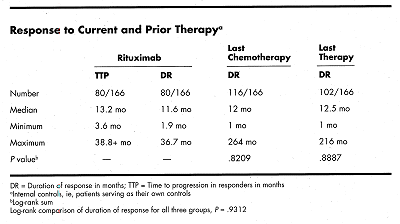
This pivotal study had internal (patient as own control) and external (literature) controls. An intent-to-treat analysis (N = 166) showed a median duration of response to last chemotherapy of 12 months compared to 11.6 months following treatment with rituximab. The Kaplan-Meier graphs overlap and there is no significant difference in duration of response (P = .072; log rank test).
The overall response rate of 48% was compared to matched literature reports for fludarabine (Fludara) and cladribine (2-CdA [Leustatin]). The overall response rate to fludarabine across four studies (N = 138) was 41% (P = .23) and to 2-CdA in two studies (N = 61) 43% (P = .46).
CONCLUSION: A short (four infusions, 22 days) outpatient course of rituximab produces responses in patients with refractory low-grade or follicular NHL of equivalent duration to prior chemotherapy. The overall response rate obtained with rituximab compares favorably to that of other single agents. Furthermore, successful retreatment with rituximab has been reported and maintenance regimens are being studied. The MoAb has shown clinical activity in first and subsequent relapses, and in refractory and bulky disease. Front-line, combination, maintenance, and other studies are in progress. Initial studies in combination with chemotherapeutic and biological agents have shown promising results. Rituximab is now being evaluated in large randomized studies in patients with intermediate/high-grade NHL.
Click here for Dr. Bruce Chesons commentary on this abstract.
Targeted Therapy First Strategy Reduces Need for Chemotherapy in Newly Diagnosed LBCL
December 7th 2025Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone may allow 57% of patients with newly diagnosed LBCL to receive less than the standard number of chemotherapy cycles without compromising curative potential.
Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.