Experts Examine Tipifarnib to Treat HNSCC With HRAS Mutations
Cesar A. Perez, MD, and Victoria M. Villaflor, MD, discuss tipifarnib in patients with head and neck cancers harboring HRAS mutations.
Victoria M. Villaflor, MD
City of Hope
Duarte, CA

Cesar A. Perez, MD
Sarah Cannon Research Institute at Florida Cancer Specialists
Orlando, FL

Although HRAS mutations occur in 4% to 8% of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC), few viable options to effectively target these oncogenic drivers are available.1
Results of a recent phase 2 study (NCT02383927) investigating the viability of tipifarnib for patients with recurrent and/or metastatic HNSCC harboring HRAS mutations may provide a rationale for its use in patients whose therapeutic options are limited.
In a Between the Lines™ conversation between Victoria M. Villaflor, MD, and Cesar A. Perez, MD, the results and key takeaways of the study, and how these may motivate future treatment, were discussed.
“Most of the patients I have seen in my practice with HRAS positivity, as far as mutations, tend to have very poor outcomes,” Villaflor, section chief of head and neck oncology and professor, Department of Medical Oncology & Therapeutics Research, City of Hope, Duarte, California, explained during the program. “They generally are your patients who progress through everything.”
Tipifarnib, a farnesyltransferase inhibitor, was developed 20 years ago but has yet to find the right patient population who derive a strong benefit, said Perez, a head and neck medical oncologist and director of drug development at Sarah Cannon Research Institute at Florida Cancer Specialists.
“It’s a drug that is at least 2 decades old and it took all this time for us to find out this specific target,” Perez continued.
Methods and Efficacy Results With Tipifarnib
A total of 22 patients with a high variant allele frequency (VAF; ≥20%) were enrolled on the trial; 20 were eligible for the efficacy analysis. The patients’ median age was 63 years (range, 20-89) and 68.2% were male.
Oral cavity was the most frequently reported site of the primary tumor in eligible patients (45.5%). Median prior lines of therapy received was 2 (range, 0-6), with all but 2 patients (90.9%) receiving first-line platinum-based therapy for locally advanced or metastatic disease.
Patients with HNSCC initially received oral tipifarnib at a dose of 900 mg 2 times a day on days 1 through 7 and 15 through 21 of 28-day cycles, but 9 of 15 patients who received this dosing required dose reductions to 600 mg twice daily in the second cycle to manage toxicities. Thus, the starting dose of tipifarnib was changed to 600 mg twice a day to improve tolerability.
“Many of the patients who had partial responses were treated with the 600-mg dose. It was a good decision from the authors of the study,” Perez explained.
The primary end point of objective response rate was 55.0% (95% CI, 31.5%-76.9%) for evaluable patients with high VAF. Partial responses were observed in 11 patients with stable disease occurring in 9, for a clinical benefit rate of 100%. A slightly higher rate of response was noted in patients with VAF >35% vs the rest of the treated patients (TABLE).
TABLE. Responses by Patient Subgroups
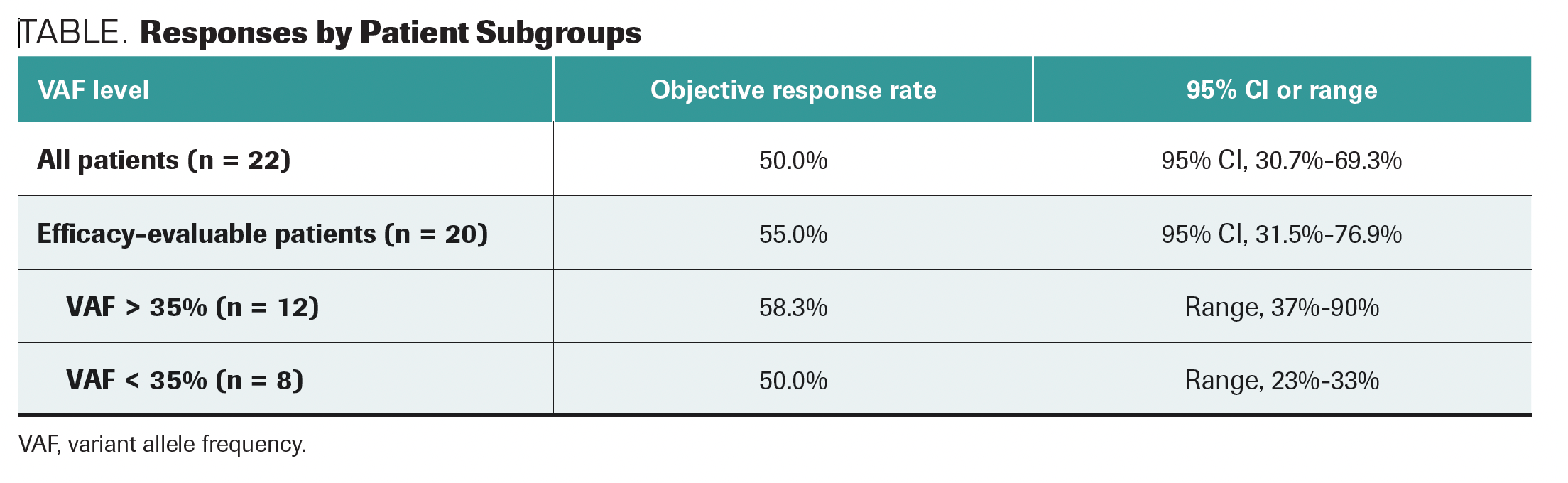
“Patients who have higher VAFs, [more than] 20%, have better durations of response to treatment,” Villaflor said.
Median progression-free survival (PFS) was higher among patients receiving tipifarnib compared with last prior therapy (P = .0012).
“In a small study, you have a [median] PFS with tipifarnib of 5.6 months [95% CI, 3.6-16.4 months] in patients who were heavily pretreated,” explained Villaflor. “Additionally, patients on their last prior therapy [had a median] PFS of 3.6 months [1.3-5.2 months], which gives you an indication of how [disease in] these patients generally behave.”
“These data [regarding] the last prior therapy are very important. As [Dr Villaflor] mentioned, we know that these are patients who were pretreated and were progressing fairly rapidly,” Perez explained. “The fact that they had close to a 6-month PFS [with tipifarnib] is very important and significant.”
In the safety analysis that featured 30 patients, hematologic adverse effects (AEs) including anemia (37%), lymphopenia (13%), neutropenia (10%), and leukopenia (10%), as well as gastrointestinal disturbances like nausea (10%) were the most common grade 3 or higher AEs.
Regarding the safety profile, Perez explained on what was observed with cytopenias. It is common, he explained, for heavily pretreated patients to experience these events even with lower treatment dosages, regardless of chosen therapy.
Treatment-emergent AEs that led to the discontinuation of tipifarnib were laryngeal obstruction in 2 patients and respiratory failure in 1. At the data cutoff point, no patients with high VAF discontinued treatment with tipifarnib due to AEs.
Future Directions With Tipifarnib in HNSCC
Given these positive data, Perez and Villaflor looked to the future for tipifarnib use in this population of patients with HNSCC and HRAS mutations.
“It seems that we finally have a target mutation that we can use in patients with HNSCC,” Perez said.
“The fact that we do have some data in a small study that show that we might be able to target this gene is very compelling. I look forward to further studies in this area, as well, to see if we can ultimately get this drug approved in this indication,” Villaflor explained.
An ongoing pivotal phase 2 study (NCT03719690) is looking at outcomes in HRAS-mutant HNSCC with tipifarnib and how HRAS mutations impact HNSCC therapies.
“Most of the head-and-neck oncology field are waiting for more data about this, and hopefully [the data] will translate into the clinic,” Perez explained.
According to Villaflor, the development of targeted therapies for patients with HNSCC has been difficult because of the interrelation of the KRAS, NRAS, and HRAS mutations, adding that the results of this research may support increased genomic testing and ultimately drive further investigation.2
“We have been studying HRAS, and all the RAS pathways, in head-and-neck cancer for a couple of decades, but it wasn’t until now that they caught it. Now that they have these data, we should try to understand [it] a bit better. We have better technology than 20 years ago, we have better sequencing, and once you understand [that], you can develop combination trials that could prolong the duration of response in these patients,” Perez explained.
References
- Ho AL, Brana I, Haddad R, et al. Tipifarnib in head and neck squamous cell carcinoma with HRAS mutations. J Clin Oncol. 2021;39(17):1856-1864. doi:10.1200/JCO.20.02903
- Pearson AT, Vokes EE. Is this the dawn of precision oncology in head and neck cancer? J Clin Oncol. 2021;39(17):1839-1841. doi:10.1200/JCO.21.00569