The population of patients with intermediate-risk prostate cancer are a large and heterogeneous group with highly variable prognoses, which present a challenge to efforts to develop standardized treatment recommendations. New classification systems have been proposed that modify the existing National Comprehensive Cancer Network guidelines and that subdivide men with intermediate-risk prostate cancer into favorable and unfavorable subgroups. This review will examine the changing landscape of intermediate-risk prostate cancer and the effects on treatment decisions that may result from this new classification. The literature provides evidence that men with favorable intermediate-risk prostate cancer have prostate cancer–specific mortality and all-cause mortality rates similar to the rates in patients with low-risk prostate cancer and thus may be candidates for active surveillance, dose-escalated radiation therapy without short-term androgen deprivation therapy (ADT), or, interestingly, standard-dose radiation therapy plus short-term ADT. Conversely, patients with unfavorable intermediate-risk prostate cancer have prostate cancer–specific mortality and all-cause mortality rates similar to the rates in patients with high-risk prostate cancer. These patients would not be candidates for active surveillance and may in fact require long-term ADT in addition to standard-dose or dose-escalated radiation therapy instead of 4 to 6 months of ADT.
Introduction
In 2015, an estimated 220,800 new cases of prostate cancer were diagnosed in the United States.[1] This figure is significant because prostate cancer–specific mortality (PCSM) is still the second leading cause of oncologic death in the United States.[1] Given the high prevalence and heterogeneous clinical behavior of prostate cancer, clinicians must differentiate indolent tumors from those that are more aggressive. Failure to differentiate can lead to overtreatment of patients with more indolent disease and undertreatment of aggressive tumors.[2-4] Risk classification systems characterize the burden of disease and help guide appropriate treatment recommendations. One classification system is the National Comprehensive Cancer Network (NCCN) risk classification system,[5] which stratifies men into very-low-, low-, intermediate-, high-, and very-high-risk groups. The risk group to which a patient is assigned is clinically significant because different treatment approaches are recommended based on the risk category.
The NCCN system defines intermediate-risk prostate cancer as having at least one of the following characteristics:
• Clinical tumor stage T2b or T2c.
• Gleason score (GS) of 7.
• Prostate-specific antigen (PSA) level of 10–20 ng/mL.
Other definitions of intermediate-risk disease have also been proposed.[6-8]
Intermediate-risk prostate cancer represents the largest of the risk groups and is comprised of a heterogeneous population of patients with variable prognoses. This heterogeneity presents a challenge to both physicians developing treatment recommendations and patients who ultimately choose a specific treatment approach. Patients within the intermediate-risk category experience a wide range of PCSM and biochemical or clinical recurrence (range, 2% to 70%) following treatment with radical prostatectomy, external beam radiation therapy (EBRT), or brachytherapy.[6,9]
In order to better understand this risk group, new classification systems have been proposed that help reduce its heterogeneity by subdividing men with intermediate-risk prostate cancer into “favorable” and “unfavorable” subgroups. This review will examine the changing landscape of intermediate-risk prostate cancer and the effects on treatment decisions that may result from the new classifications. It should be noted that a detailed examination of the role of brachytherapy in intermediate-risk prostate cancer is under study and beyond the scope of this review.
Favorable vs Unfavorable Intermediate-Risk Prostate Cancer
The D’Amico risk groups,[6] initially published in 1998, were designed to stratify patients according to the likelihood of biochemical recurrence–free survival after radical prostatectomy or radiotherapy. The current NCCN guidelines are a slight modification of this classification system. However, in 2005 an International Society of Urological Pathology conference was held in order to reach a consensus regarding the grading of prostate cancer. A consensus statement was published in 2005,[10] and as a result of the adoption of this new grading system, the reporting of secondary pattern Gleason grade 4 disease became more prevalent. Several investigators have reported on their observation of grade migration from GS 3+3 to GS 3+4 (indicating primary pattern 3 disease but with a lesser amount of pattern 4).[11-14] This grade migration could cause a number of men who previously would have been categorized as low-risk to be assigned to the NCCN intermediate-risk category because of their GS, thereby improving the prognosis of both groups (the Will Rogers effect). Thus, it has been hypothesized that some men with GS 3+4 intermediate-risk prostate cancer may have a low risk of PCSM and higher rates of overall survival (OS), similar to what is seen in patients with low-risk prostate cancer.[15]
Historically, prostate cancer risk prediction models and observational studies that have adjusted for GS utilized only the total GS.[16,17] Numerous studies suggest that not all Gleason scores of 7 are created equal, and that GS 3+4 tumors have a better prognosis than GS 4+3 tumors.[18-23] In 2009, Stark and colleagues published their research, which was based on three study pathologists’ blinded standardized review of 693 prostatectomy specimens and 119 biopsy specimens in order to assign primary and secondary Gleason patterns.[24] The researchers collected 20 years of follow-up data on these patients. They found that prostatectomy patients with a standardized GS of 4+3 were 3.1 times more likely to develop lethal prostate cancer than patients with a GS of 3+4 (95% CI, 1.1–8.6). They also reported crude cancer mortality rates per 1,000 person-years of 10.8 for GS 3+4 disease and 45.2 for GS 4+3.
Reese and colleagues analyzed the heterogeneity of the NCCN prostate cancer risk groups by investigating whether the outcomes of patients who had undergone radical prostatectomy differed among patients within the same risk group, depending on which risk criteria were present.[25] Included in the cohort of men they studied were 4,164 with intermediate-risk prostate cancer. Within this group, the biochemical recurrence–free survival rates differed significantly, according to the number of risk factors present. For patients with one intermediate-risk factor, the 5-year biochemical recurrence–free survival was 83.0%, compared with 64.3% for men with two risk factors and 45.9% for those with three risk factors (P < .01). There was no significant difference in biochemical recurrence–free survival between low-risk men and those classified as intermediate-risk because of clinical stage. Similarly, the biochemical recurrence–free survival was similar between intermediate-risk men and those classified as high-risk because of their clinical stage.
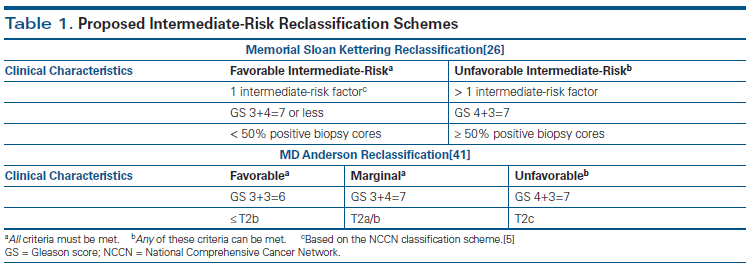
In 2012, Zumsteg and Zelefsky studied the variability in prognosis within intermediate-risk prostate cancer, as illustrated by two patients presenting with intermediate-risk disease.[26] The first was an 85-year-old man with clinical stage T1c prostate cancer, a GS of 3+4=7 in 1 of 12 biopsy cores, and a PSA level of 3.0 ng/mL. The second was a 45-year-old man with clinical stage T2c prostate cancer, a GS of 4+3=7 in 12 of 12 cores, and a PSA level of 19 ng/mL. Using the Memorial Sloan Kettering Cancer Center prognostic nomogram,[27] they found that the 85-year-old man would have an 82% probability of biochemical recurrence–free survival at 10 years after EBRT alone, compared with a 40% probability of biochemical recurrence–free survival for the 45-year-old man, also treated with EBRT alone. The authors argued that a one-size-fits-all treatment algorithm based purely on risk classification might not be the most appropriate approach. With this heterogeneity in mind, they categorized intermediate-risk patients into favorable and unfavorable subgroups, based on their clinical characteristics (Table 1). Favorable patients were those who had all of the following:
• Only one intermediate-risk factor (based on the NCCN classification scheme).
• GS of 3+4=7 or less.
• Less than 50% of biopsy cores positive for cancer.
Those who were classified as unfavorable could have any of the following:
• More than one intermediate-risk factor.
• GS of 4+3=7.
• Greater than 50% positive biopsy cores.[28]
Additionally, Zumsteg and Zelefsky proposed a risk-adapted treatment strategy based on their interpretation of the available data for these intermediate-risk patients. They suggested that dose-escalated radiation therapy (DERT) alone might be sufficient treatment for patients with favorable intermediate-risk (FIR) disease. However, they suggested that DERT along with 4 to 6 months of androgen deprivation therapy (ADT) should be considered the standard of care for men with unfavorable intermediate-risk (UIR) prostate cancer. Lastly, they stated that the addition of short-term ADT for patients with unfavorable features could be considered on the basis of extrapolation of data from trials of EBRT.
Validating This New Classification System
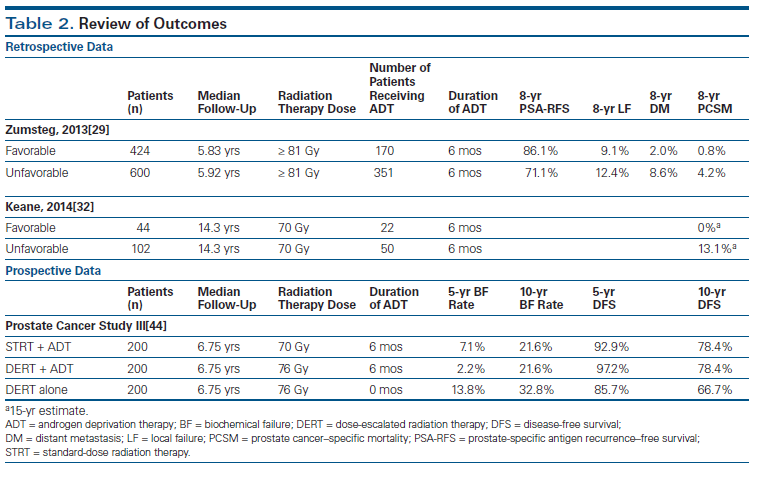
In order to validate the new classification system, the same authors retrospectively reviewed 1,024 men with intermediate-risk prostate cancer who underwent definitive DERT, defined as ≥ 81 Gy.[29] They evaluated biochemical recurrence–free survival, incidence of distant metastasis, and PCSM in patients classified as FIR or UIR. They also examined the effect of ADT on the aforementioned endpoints. The investigators reported that primary Gleason pattern 4 (hazard ratio [HR], 3.26; P < .0001), percent positive biopsy cores ≥ 50% (HR, 2.72; P = .0007), and multiple intermediate-risk factors (HR, 2.20; P = .008) were all significant predictors of increased distant metastasis in multivariate analyses. Primary Gleason pattern 4 (HR, 5.23; P < .0001) and percent positive biopsy cores ≥ 50% (HR, 4.08; P = .002) both independently predicted an increased PCSM. They also reported that men with UIR disease had inferior biochemical recurrence–free survival (HR, 2.37; P < .0001), distant metastasis (HR, 4.34; P = .0003), and PCSM (HR, 7.39; P = .007) compared with those with FIR disease, despite the fact that UIR patients were more likely to receive ADT (Table 2). Interestingly, they also found no difference in outcome between FIR patients and 511 low-risk patients treated with radiation doses of at least 81 Gy in terms of biochemical recurrence–free survival (P = .142), distant metastasis (P = .693), or PCSM (P = .697).
When examining the effect of ADT on these two groups of patients, they found that patients with FIR prostate cancer had a significant prolongation of 8-year biochemical recurrence–free survival with ADT (93.6% vs 80.9%; P = .001) but no significant difference in 8-year distant metastasis (0% vs 3.3%; P = .125) or 8-year PCSM (0% vs 1.3%; P = .450). In contrast, ADT for patients with UIR prostate cancer significantly improved 8-year biochemical recurrence–free survival (75.1% vs 65.3%; P = .002), distant metastasis (6.4% vs 10.6%; P = .045), and PCSM (2.2% vs 7.2%; P = .013). The authors also found that patients with multiple unfavorable risk factors had significantly decreased 8-year biochemical recurrence–free survival (60.3% vs 73.7%; P = .001) and increased local failure (24.2% vs 9.7%; P = .024), distant metastasis (22.9% vs 5.2%; P < .001), and PCSM (10.5% vs 2.7%; P < .001) compared with those with only one unfavorable risk factor. Lastly, they found no significant difference in outcome between intermediate-risk patients with multiple unfavorable risk factors and 582 high-risk patients treated with EBRT doses of at least 81 Gy along with long-term ADT-in terms of biochemical recurrence–free survival (P = .198), distant metastasis (P = .523), or PCSM (P = .738).
Based on these results, the authors concluded that in the dose-escalation era, intermediate-risk prostate cancer is a heterogeneous disease that should be stratified into favorable and unfavorable groups. They showed that these risk subgroups have markedly different prognoses, with UIR prostate cancer having a 2.4-fold increase in biochemical recurrence, a 4.3-fold increase in distant metastasis, and a 7.4-fold increase in PCSM, despite UIR patients being twice as likely to receive ADT as a part of their therapy. They proposed the omission of short-term ADT as a potential option for patients with FIR disease undergoing DERT, particularly older men or patients with cardiac comorbidities,[30,31] but noted that this proposal should be investigated further in prospective trials. The authors suggested that patients with multiple UIR factors might be treated with a regimen similar to that used in patients with high-risk disease, including long-term ADT. However, patients with only a single unfavorable risk factor might constitute a cohort that could be effectively treated with short-term ADT and DERT. While Zumsteg and Zelefsky’s data are promising, it is important to recall that this study was retrospective in nature; the authors’ suggestions should thus be viewed with caution.
Prostate Cancer–Specific Mortality
Keane and colleagues[32] utilized data from a prospective randomized trial to better assess the long-term outcomes of men with intermediate-risk prostate cancer. The authors used the Zumsteg definitions of unfavorable and favorable disease.[29] They also compared the UIR patients in this trial with men who had high-risk prostate cancer, and they evaluated the risk of PCSM in a competing-risks analysis, adjusting for age, comorbidity, and treatment. This prospective trial randomized patients to 3-dimensional (3D) conformal radiation therapy (3DCRT) to 70 Gy with or without 6 months of ADT (ClinicalTrials.gov identifier: NCT00116220). Median follow-up was 14.3 years. There were no deaths due to prostate cancer in the FIR group. There was an increase in PCSM among men with high-risk prostate cancer when compared with the UIR group, but the difference was not significant (HR, 1.59 [95% CI, 0.66–3.83]; P = .30) after adjusting for age, randomized treatment arm, and comorbidity. The 15-year estimates of PCSM were 20.05% (95% CI, 8.98%–34.26%), 13.10% (95% CI, 6.96%–21.21%), and 0% (95% CI, 0%–0%) for patients who had high-risk, UIR, and FIR prostate cancer, respectively (see Table 2). Given that men with UIR prostate cancer had a PCSM similar to that of men with high-risk prostate cancer, the authors hypothesized that some UIR patients may harbor occult prostate cancer with a GS from 8 to 10 and may potentially benefit from additional staging with a multiparametric magnetic resonance imaging (MRI) scan and targeted biopsy to rule this out-as well as benefiting from long-term ADT; they noted that this was particularly true in those UIR patients with a percent of positive biopsy cores ≥ 50% and/or multiple intermediate-risk factors. They also suggested that men with FIR prostate cancer may not require ADT in addition to radiation therapy (RT), echoing what Zumsteg and colleagues suggested in their analysis. Interestingly, the authors also suggested that active surveillance (AS) might be an appropriate option for men who have FIR prostate cancer and severe comorbidities, but they noted that this required further study.
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"46879","attributes":{"alt":"","class":"media-image","id":"media_crop_509966181477","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"5474","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","title":" ","typeof":"foaf:Image"}}]]
James B. Yu, MD, MHS
Yale School of Medicine
New Haven, ConnecticutWhether to Include ADT for Intermediate-Risk Disease: There’s More to the Decision Than Just DataThe impetus for investigating radiotherapy alone (without androgen deprivation therapy [ADT]) for intermediate-risk disease lies, of course, in patient preference. ADT is, in my opinion, much more difficult and bothersome than is typically portrayed by physician-graded toxicity, and it has been shown to be associated with patient regret regarding their decision to undergo prostate cancer treatment. Fatigue, hot flashes, weight gain, and loss of libido and vigor are only a few of the side effects reported to me in my clinic. These side effects may not rise to the level of a “grade 3” toxicity, but they are the types of side effects that still weigh heavily on the minds of my patients-even when “short-term.”Advances in Biopsy Techniques and Molecular Biology May Add to the ConfusionEven after the Radiation Therapy Oncology Group 0815 and ProtecT trials are reported, controversy will continue as we struggle with how to incorporate information from targeted prostate biopsies, as well as improvements in the molecular classification of prostate cancer. If a finding of greater than 50% positive biopsy cores is prognostic of unfavorable intermediate-risk (UIR) disease with standard biopsy, how do we assess the percent positivity of a magnetic resonance imaging–guided biopsy? If an otherwise UIR prostate cancer has molecular markers predictive of good radiotherapy response, should ADT be omitted?Always present will be the need to incorporate increasing amounts of data (thankfully, we will have excellent reviews such as this one to help), and the need for shared decision-making based on a patient’s needs, hopes, and fears.
ADT for Intermediate-Risk Prostate Cancer
ADT along with EBRT, rather than EBRT alone, has become a standard-of-care treatment option for patients with intermediate-risk disease, based on multiple prospective randomized studies that demonstrated improved outcomes with the addition of ADT to conventional-dose radiation (65–70 Gy).[30,33,34] However, since the completion of these studies, intensity-modulated RT (IMRT) and image-guided therapy have improved the precision and accuracy of EBRT. Additionally, there are prospective randomized trials that have shown improvement in biochemical recurrence–free survival with an escalating dose to 76–79.2 Gy, and these higher doses are now the current standard of care.[35,36] Furthermore, ADT is associated with a number of toxicities, including increased risks of cardiovascular disease, dyslipidemia, obesity, erectile dysfunction, and osteoporosis.[31,37-39] Thus, in the dose-escalated era, the benefit of ADT in intermediate-risk prostate cancer is less clear.
Zapatero and colleagues[40] recently published data from a phase III randomized trial that examined ADT duration in intermediate- and high-risk patients treated with DERT. One hundred seventy-eight patients (81 with intermediate risk) were randomly assigned to receive short-term ADT, and 177 (85 with intermediate risk) to receive long-term ADT. The authors found statistically significant improvements in 5-year biochemical recurrence–free survival, 5-year OS (P = .009), and 5-year metastasis-free survival (P = .01) favoring the long-term ADT arm. However, their planned subset analysis revealed that the benefit was largely in the high-risk patients, with no statistically significant improvements in outcomes in the intermediate-risk patients. The authors did not stratify the intermediate-risk patients but did acknowledge that with longer follow-up they hoped to do so, in order to better clarify whether any intermediate-risk patients benefit from long-term ADT in the dose-escalated era.
To address the relative contributions of higher-dose RT and ADT, Castle and colleagues[41] subclassified intermediate-risk patients based on the risk of disease recurrence when treated with RT alone and then determined the benefit of adding ADT within each of the intermediate-risk subsets. Three groups of men were retrospectively analyzed in this study, including 326 intermediate-risk patients treated with RT alone, 218 intermediate-risk patients treated with RT and ≤ 6 months of ADT, and 274 low-risk patients treated with definitive RT. All patients were treated with IMRT or 3DCRT to 75.6–78 Gy; the median follow-up was 58 months. Recursive partitioning analysis was performed, and intermediate-risk patients treated with RT alone were divided into three prognostic groups: 188 favorable patients (GS of 6, ≤ T2b; or GS of 3+4, ≤ T1c); 71 marginal patients (GS of 3+4, T2a/b); and 68 unfavorable patients (GS of 4+3 or T2c disease) (see Table 1). It should be noted that this classification system differs from that of Zumsteg et al.[29] The favorable subset was used as the reference group (HR, 1.0), and HRs for the marginal and unfavorable groups were 2.1 and 4.6, respectively. Among the intermediate-risk subsets treated with RT alone, freedom from failure (FFF) at 5 years was 94% for the favorable group, 91% for the marginal group, and 74% for the unfavorable group (P = .0002). When looking at the effect of ADT, the authors found that in the unfavorable subset, FFF at 5 years with RT alone was 74%, compared with 94% for those treated with RT and ADT (P = .0049). For patients in the marginal subset, FFF at 5 years with RT alone was 91%, compared with 100% for those who received combined-modality treatment (P = .076). The favorable subset had nearly identical outcomes whether treated with RT or RT plus ADT (FFF at 5 years, 94% and 95%, respectively; P = .8546). They also found that the patients in the favorable subgroup treated with RT alone had a FFF at 5 years that was close to that of a similar cohort of low-risk patients treated with RT alone (94% vs 98%; P = .0596). Thus, the authors concluded that men with FIR prostate cancer may not benefit from ADT when combined with DERT; their FFF was nearly as good as that of patients with low-risk disease in this retrospective analysis. However, in men with a GS of 4+3 or T2c disease, the addition of ADT to DERT did improve FFF.
Can ADT Compensate for Dose Escalation?
To better identify which patients would benefit from ADT, Stoyanova and colleagues[42] developed prediction tools to assist physicians and patients in estimating the potential gains in biochemical control from adding ADT to DERT or standard-dose RT (SDRT; 65–70 Gy). The authors examined 3,215 low-risk, intermediate-risk, and high-risk patients with clinically localized prostate cancer who received definitive EBRT with or without ADT. Two nomograms (one with ADT and one without ADT) were created in order to develop these prediction tools. The authors found that their nomograms accurately predicted the probability of biochemical failure at 8 years after RT. The model included the percentage of tumor cells with a Gleason pattern of 4 or 5,[43] the positive percentage of biopsy cores, the pretreatment PSA level, ADT duration, and RT dose as continuous covariates. T-stage was used as a categorical variable, and GS was used as a categorical variable in the no-ADT nomogram and as a continuous variable in the ADT nomogram. The authors provided examples in order to teach clinicians and patients how to use their nomograms. For instance, a patient with a T1/T2 tumor, GS of 7, pretreatment PSA level of 10 ng/mL, 5% of cells with a Gleason pattern of 4 or 5, and a positive percentage of biopsy of 10% would be expected to experience a reduction in the 8-year risk of biochemical failure from 40% to 35% if his RT dose were increased from 70 Gy to 80 Gy. However, adding 6 months of ADT would reduce the biochemical failure rate to 20% for 70 Gy and to 18% for 80 Gy. The authors concluded that the data from these nomograms suggested that, with regard to reducing biochemical failure, the gains were far greater when ADT was added than when the RT dose was increased from 70 Gy to 80 Gy. After applying the nomogram to hypothetical patients, they observed that for most patients, short-term (≤ 6 months) to intermediate-term (from > 6 months to < 2 years) ADT would be favored over extending ADT to 2 years or more.
The Prostate Cancer Study III examined the addition of ADT to SDRT and DERT in intermediate-risk patients (ClinicalTrials.gov identifier: NCT00223145). The preliminary results of this trial have now been published in abstract form.[44] A total of 600 patients were enrolled. Intermediate-risk prostate cancer was defined as T1/T2 disease, GS ≤ 6, PSA level 10–20 ng/mL; or T1/T2 disease, GS of 7, PSA level ≤ 20 ng/mL. Patients were randomly assigned to one of three arms: 6 months of ADT plus 70 Gy to the prostate (arm 1), 6 months of ADT plus 76 Gy (arm 2), or 76 Gy alone (arm 3). ADT consisted of bicalutamide and goserelin for 6 months. RT was delivered using a 3D conformal technique and started 4 months after the beginning of ADT. Median follow-up was 6.75 years. Primary endpoints were biochemical failure and disease-free survival (DFS). Secondary endpoints included OS, as well as hormonal and radiation-related toxicities. Biochemical failure was defined as 2 ng/mL above the PSA nadir.
Two hundred patients were enrolled in each of the three arms. The 5-year biochemical failure rates were 7.1%, 2.2%, and 13.8% for arms 1, 2, and 3, respectively; the 10-year biochemical failure rates were 21.6%, 21.6%, and 32.8% for arms 1, 2, and 3, respectively. Significant differences in biochemical failure rates were observed at 5 and 10 years between arms 1 and 3 (P = .024; P = .023) and between arms 2 and 3 (P < .001; P = .002). However, no significant differences at 5 or 10 years were observed between arms 1 and 2. The 5-year DFS rates for the three arms were 92.9%, 97.2%, and 85.7%, respectively; the 10-year DFS rates for the three arms were 78.4%, 78.4%, and 66.7%, respectively (see Table 2). Significant differences in DFS were observed at 5 and 10 years between arms 1 and 3 (P = .016; P = .016) and between arms 2 and 3 (P < .001; P = .001). However, DFS differences between arms 1 and 2 were not significant. There were 137 patients who died (22.8%), but only 8 deaths (1.3%) were attributed to prostate cancer. The 5-year OS rates for the three arms were 90.5%, 93.7%, and 91.0%, respectively; the 10-year OS rates for the three arms were 63.3%, 72.2%, and 74.7%, respectively. There was no statistical difference in OS among the three treatment arms.
With regard to radiation toxicity in arm 1 (70 Gy), as compared with arms 2 and 3 (76 Gy), there was no significant difference in acute gastrointestinal (GI) toxicity (7.8% vs 8.1%; P = .88); in acute genitourinary (GU) toxicity (31.1% vs 29.3%; P = .65); or in late GU toxicity (20.1% vs 18.4%; P = .712). However, there was a significant difference in late GI toxicity (5.1% vs 15.8%; P < .001). Thus, the authors concluded that adding ADT to moderate-dose RT significantly improved biochemical failure and DFS, with a better late GI toxicity profile compared with DERT alone. The data are quite provocative, as they suggest no need to escalate RT doses beyond 70 Gy in this patient population, supporting the findings of Stoyanova and colleagues, but in stark contrast to practice patterns in the United States, where doses at or above 75.6 Gy are routinely used. As provocative as this may be, the results of this study are still available in abstract form only, and a cautious approach to changing practice patterns is advised until the full results of this prospective trial are available and their significance understood.
Active Surveillance
AS is a viable approach for patients with very-low-risk or low-risk prostate cancer, even in those with a life expectancy of at least 10 years, based on current NCCN guidelines.[5] AS involves monitoring the course of prostate cancer, with the expectation that curative treatment will be initiated if the cancer progresses.[45,46] Given the aforementioned grade migration and the possibility of “lower-risk” men being included in the NCCN intermediate-risk category, AS may also be an appropriate initial option for men with FIR prostate cancer.
To date, no randomized data comparing AS with definitive local therapy in intermediate-risk prostate cancer have been published. However, a number of prospective phase II studies[47-50] have included patients with intermediate-risk disease. One of the largest and most recently updated phase II trials was performed by Klotz and colleagues.[51] The main outcomes measured in the treated patients were OS, disease-specific survival, rate of treatment, and PSA failure rate. In 2015, the authors reanalyzed 993 patients. Twenty-one percent of the patients had intermediate-risk prostate cancer, and 132 had GS 3+4 disease. One hundred forty-nine of the patients (15%) died, and 844 were still alive at the time of update. There were 15 deaths from prostate cancer (1.5%); the 10-year and 15-year actuarial cause-specific survival rates were 98.1% and 94.3%, respectively, even with 21% of the patients having intermediate-risk disease. Given this low 15-year prostate cancer mortality, the authors concluded that screened men older than age 70 years with intermediate-risk prostate cancer may be candidates for AS. While these data are promising, we await the results from the randomized phase III UK ProtecT trial (ClinicalTrials.gov identifier: NCT02044172),[52] which is comparing AS with definitive local therapy, and which has included men with FIR prostate cancer.
Conclusions and Future Directions
Risk classification systems for prostate cancer set out to characterize the burden of disease for a specific patient and help guide appropriate treatment recommendations. However, within risk categories are heterogeneous patient populations that may benefit from more tailored treatment instead of a one-size-fits-all approach; this is particularly true of the intermediate-risk group. To aid in individualization of treatment, subclassifications are emerging that categorize patients into FIR and UIR groups. The studies reviewed here suggest that men with FIR prostate cancer may have PCSM and all-cause mortality rates similar to those of low-risk prostate cancer patients and thus may be candidates for AS, DERT without short-term ADT, or, interestingly, SDRT plus short-term ADT. Conversely, these studies have suggested that patients with UIR disease have PCSM and all-cause mortality rates similar to those of high-risk prostate cancer patients. These UIR patients would certainly not be candidates for AS and might in fact require long-term ADT in addition to SDRT or DERT. However, level 1 evidence supporting these preliminary results is required before major changes in treatment paradigms can be recommended.
Additional data from prospective trials are on the horizon that may help clarify the heterogeneity in intermediate-risk disease. The ProtecT trial[52] randomly assigned patients with FIR prostate cancer to either AS or definitive local therapy (radical prostatectomy or EBRT). More than 1,600 men with varying risks of disease were enrolled; the initial results are expected to be reported within the next few years.
In the United States, the Radiation Therapy Oncology Group 0815 trial (ClinicalTrials.gov identifier: NCT00936390) will hopefully better clarify which intermediate-risk prostate cancer patients need ADT when treated with DERT. This study is currently enrolling intermediate-risk patients and randomly assigning them either to 79.2 Gy of EBRT plus 6 months of ADT or to DERT alone. The study will provide data regarding short-course ADT and the risk of PCSM in men with FIR and UIR prostate cancer who are receiving DERT. However, it will not provide insight into whether adding long-term ADT to DERT is required to minimize the risk of PCSM in men with UIR prostate cancer. This issue requires further study.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Fast Stats. http://seer.cancer.gov/faststats. Accessed January 20, 2016.
2. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117-23.
3. Raldow AC, Presley CJ, Yu JB, et al. The relationship between clinical benefit and receipt of curative therapy for prostate cancer. Arch Intern Med. 2012;172:362-3.
4. Swisher-McClure S, Pollack CE, Christodouleas JP, et al. Variation in use of androgen suppression with external-beam radiotherapy for nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83:8-15.
5. Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686-718.
6. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969-74.
7. Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491-500.
8. Chism DB, Hanlon AL, Horwitz EM, et al. A comparison of the single and double factor high-risk models for risk assignment of prostate cancer treated with 3D conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:380-5.
9. Mitchell JA, Cooperberg MR, Elkin EP, et al. Ability of 2 pretreatment risk assessment methods to predict prostate cancer recurrence after radical prostatectomy: data from CaPSURE. J Urol. 2005;173:1126-31.
10. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228-42.
11. Albertsen PC. What is the risk posed by prostate cancer? J Natl Cancer Inst Monogr. 2012;2012:169-74.
12. Helpap B, Egevad L. The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Archiv. 2006;449:622-7.
13. Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248-53.
14. Gallina A, Chun FK, Suardi N, et al. Comparison of stage migration patterns between Europe and the USA: an analysis of 11 350 men treated with radical prostatectomy for prostate cancer. BJU Int. 2008;101:1513-8.
15. Raldow AC, Zhang D, Chen MH, et al. Risk group and death from prostate cancer: implications for active surveillance in men with favorable intermediate-risk prostate cancer. JAMA Oncol. 2015;1:334-40.
16. Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445-51.
17. Stephenson AJ, Kattan MW. Nomograms for prostate cancer. BJU Int. 2006;98:39-46.
18. Chan TY, Partin AW, Walsh PC, Epstein JI. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823-7.
19. Rasiah KK, Stricker PD, Haynes AM, et al. Prognostic significance of Gleason pattern in patients with Gleason score 7 prostate carcinoma. Cancer. 2003;98:2560-5.
20. Sengupta S, Slezak JM, Blute ML, et al. Trends in distribution and prognostic significance of Gleason grades on radical retropubic prostatectomy specimens between 1989 and 2001. Cancer. 2006;106:2630-5.
21. Kang DE, Fitzsimons NJ, Presti JC Jr, et al. Risk stratification of men with Gleason score 7 to 10 tumors by primary and secondary Gleason score: results from the SEARCH database. Urology. 2007;70:277-82.
22. Khoddami SM, Shariat SF, Lotan Y, et al. Predictive value of primary Gleason pattern 4 in patients with Gleason score 7 tumours treated with radical prostatectomy. BJU Int. 2004;94:42-6.
23. Lau WK, Blute ML, Bostwick DG, et al. Prognostic factors for survival of patients with pathological Gleason score 7 prostate cancer: differences in outcome between primary Gleason grades 3 and 4. J Urol. 2001;166:1692-7.
24. Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459-64.
25. Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80:1075-9.
26. Zumsteg ZS, Zelefsky MJ. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: the standard of care? Lancet Oncol. 2012;13:e259-e269.
27. Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133-9.
28. D’Amico AV, Renshaw AA, Cote K, et al. Impact of the percentage of positive prostate cores on prostate cancer-specific mortality for patients with low or favorable intermediate-risk disease. J Clin Oncol. 2004;22:3726-32.
29. Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895-902.
30. D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289-95.
31. Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448-56.
32. Keane FK, Chen MH, Zhang D, et al. The likelihood of death from prostate cancer in men with favorable or unfavorable intermediate-risk disease. Cancer. 2014;120:1787-93.
33. Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841-50.
34. Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107-18.
35. Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67-74.
36. Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990-6.
37. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154-64.
38. Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181-6.
39. O’Farrell S, Garmo H, Holmberg L, et al. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243-51.
40. Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320-7.
41. Castle KO, Hoffman KE, Levy LB, et al. Is androgen deprivation therapy necessary in all intermediate-risk prostate cancer patients treated in the dose escalation era? Int J Radiat Oncol Biol Phys. 2013;85:693-9.
42. Stoyanova R, Pahlajani NH, Egleston BL, et al. The impact of dose-escalated radiotherapy plus androgen deprivation for prostate cancer using 2 linked nomograms. Cancer. 2013;119:1080-8.
43. D’Ambrosio DJ, Hanlon AL, Al-Saleem T, et al. The proportion of prostate biopsy tissue with Gleason pattern 4 or 5 predicts for biochemical and clinical outcome after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1082-7.
44. Nabid A, Carrier N, Vigneault E, et al. Place of short-term androgen deprivation therapy in intermediate-risk prostate cancer treated with radiotherapy: a phase III trial. J Clin Oncol. 2015;33(suppl 7):abstr 5.
45. Thomsen FB, Brasso K, Klotz LH, et al. Active surveillance for clinically localized prostate cancer-a systematic review. J Surg Oncol. 2014;109:830-5.
46. Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28:2810-6.
47. Klotz L. Active surveillance: the Canadian experience with an “inclusive approach”. J Natl Cancer Inst Monogr. 2012;2012:234-41.
48. Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63:101-7.
49. Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol. 2013;64:981-7.
50. Bul M, van den Bergh RC, Zhu X, et al. Outcomes of initially expectantly managed patients with low or intermediate risk screen-detected localized prostate cancer. BJU Int. 2012;110:1672-7.
51. Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272-7.
52. Lane JA, Donovan JL, Davis M, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014;15:1109-18.