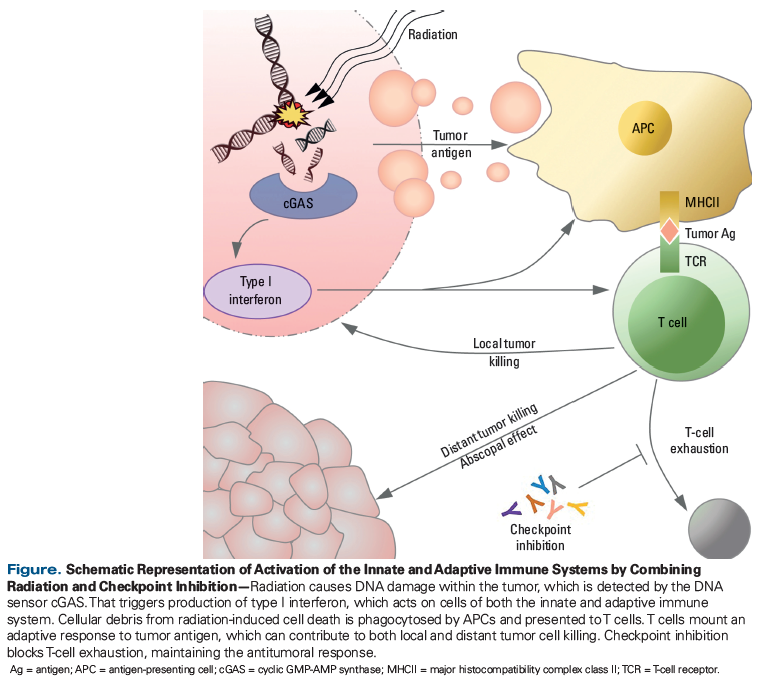
In this article, we discuss the immunogenicity of radiation-induced cell death and describe the innate immune signaling pathways that precede adaptive antitumoral immunity. The innate and adaptive immune systems work in concert to generate systemic immune responses. In the setting of cancer, DNA damage caused by radiotherapy activates the innate immune system while tumor cell death liberates antigen that serves as a target for adaptive immunity. The immunomodulatory effects of radiation have been investigated in preclinical models; here we summarize the available data, with particular attention to the effects of radiotherapy timing, location, dose, and fractionation strategy on the antitumoral immune response. We synthesize preclinical and clinical information regarding the potential superiority of hypofractionated radiation for induction of proinflammatory responses. Although many questions remain, early successes with combining immunotherapy and radiotherapy merit further inquiry into the dose and fractionation strategies best able to activate and sustain an antitumoral response.
Introduction
Since x-rays were first used to treat cancer in the 1890s,[1] the field of radiation oncology has evolved clinically toward dose and fractionation strategies that maximize tumor control while minimizing side effects. Most of these side effects-inflammation, fibrosis, necrosis-are immunologically mediated. As we have learned more about the way cells die, and the way the immune system preserves self-tolerance to protect us from autoimmunity while fighting foreign pathogens, it has become clear that cell death occurs on a spectrum from immunogenic to tolerogenic. Current clinical dose and fractionation strategies likely lie in the middle of this spectrum because we have empirically selected treatment regimens over decades for the express purpose of killing tumor cells while minimizing attendant inflammation.
Recent successes in combining immunotherapy and radiotherapy have prompted consideration of the role radiation might play in augmenting the antitumoral immune response. Because radiation can kill cells and liberate tumor antigen, it functions as a complete vaccine: both antigen and adjuvant.[2] In the setting of checkpoint inhibition, which removes barriers to activation of the adaptive immune response, all the ingredients of a successful immunologic defense are present: innate immune activation lays the groundwork for a T-cell–mediated systemic defense. In this article, we discuss radiation’s immunomodulatory effects, with particular attention to the impact of dose and fractionation on the antitumoral response.
Radiation-Induced Cell Death: Tolerogenic, Immunogenic, or Both?
The immune system is an antimicrobial arsenal, curated by evolution, and challenged not only with responding effectively to threats from the environment, but also with preserving self-tolerance. It has two arms that work in concert: the innate immune system, which acts quickly to protect the host from imminent danger; and the adaptive immune system, which is capable of “learning” the nature of a threat and mounting a specific defense. Defending the self too robustly can be as deadly to the host as no defense at all. The problem of self/nonself discrimination is central to understanding the immunogenicity of cell death. In normal physiologic conditions, cellular turnover is estimated to be 50 to 70 billion cells per day, with the majority of these cells dying via apoptosis.[3] By necessity, apoptotic cell death is nonimmunogenic or actively tolerogenic, given the daily burden that homeostatic cellular turnover places on clearance mechanisms.[4]
However, there are circumstances in which cell death should elicit an immune response, such as injury to or infection of the host. The most extensively studied forms of proinflammatory cell death are necrosis, necroptosis, pyroptosis, and autophagic cell death.[5-7] While a full description of these processes is beyond the scope of this article, these forms of cellular demise activate the innate immune system, setting the stage for a subsequent adaptive response. How, then, do cells die following irradiation? Mitotic catastrophe and cellular senescence are induced by radiation, but these terms refer to the loss of proliferative capacity, not to the cessation of all cellular processes. True cellular demise involves a loss of morphologic and genomic integrity, followed by a confrontation with the immune system, eliciting a tolerogenic or immunogenic response, depending on the manner of death. Our focus here will be on two mechanisms by which radiation can promote an inflammatory response.
1. AIM2 recognition of DNA damage
In murine models involving exposure to high-dose radiation, innate immune signaling pathways activated by DNA damage have been shown to proceed through cytosolic DNA sensors. AIM2 (absent in melanoma 2) is a cytoplasmic DNA sensor that recognizes double-stranded DNA damaged by radiation. Activation of AIM2 causes assembly of the inflammasome, which results in activation of caspase 1.[8] Activated caspase 1 triggers release of proinflammatory cytokines and promotes the proinflammatory form of cell death known as pyroptosis.[9]
2. cGAS recognition of DNA damage, with activation of STING
Recent work by Vanpouille-Box et al demonstrated the activation of a second innate immune pathway, which depends on the adaptor protein STING (stimulator of interferon genes).[10] They showed that in the presence of a checkpoint inhibitor and a radiotherapy regimen of 8 Gy × 3, the cytosolic DNA sensor cGAS (cyclic GMP-AMP synthase) recognizes damaged DNA and activates STING, which results in the recruitment of dendritic cells, and the priming of antitumoral CD8+ T cells. This dose and regimen reliably results in tumor responses both within and distant from the radiation field, with the latter off-target response termed the abscopal effect (depicted in the Figure). At doses higher than 8 Gy × 3, the DNA exonuclease TREX1 (3’ repair exonuclease 1) is activated, degrades the cytoplasmic DNA, and prevents recruitment of dendritic cells and the subsequent priming of T cells, which eliminates the immunologically mediated response against tumors outside the initial radiation field.[10]
Synergy Between Radiotherapy and Immunotherapy
AAs noted above, the abscopal effect refers to the regression of tumor distant from a treated site. Historically, the physiologic underpinnings of this phenomenon were unknown, but work over the past decade has shown the effect to be immunologically mediated.[11] Particular attention has been paid to abscopal responses documented in case reports, which describe robust off-target effects in patients receiving immune checkpoint inhibitors who also are given radiation. A recent systematic review of case reports of the abscopal effect identified 46 reported cases in the literature.[12] Of particular note is a study in the New England Journal of Medicine by Postow et al, describing a 33-year-old woman with metastatic melanoma.[13] While being treated with ipilimumab, she received palliative radiation to a pleural soft-tissue mass, which was treated to 28.5 Gy (9.5 Gy × 3 fractions). When a 1-month follow-up scan showed no evidence of response, she was given another cycle of ipilimumab. The patient’s 4-month follow-up scan showed a dramatic response in the radiated lesion, as well as responses at her other sites of disease: hilar and splenic lesions. Her response correlated with the production of antibodies against the NY-ESO-1 (New York esophageal squamous cell carcinoma–1) melanoma protein and an increase in the number of NY-ESO-1–specific interferon γ–producing CD4+ T cells, indicating that she had mounted an adaptive immune response to tumor antigen.
The most dramatic examples of the abscopal effect have occurred in the presence of immune checkpoint inhibitors, likely because checkpoint inhibition reduces the barrier to creating an engaged and sustained adaptive immune response. The conceptual appeal of synergy between radiation and immunotherapy prompted a secondary analysis of the KEYNOTE-001 trial, which examined disease control in patients who had received radiotherapy prior to receiving pembrolizumab. The original KEYNOTE-001 trial enrolled 98 patients between 2012 and 2014, with the goal of studying pembrolizumab’s safety and efficacy, but 42 of the 98 patients had undergone previous radiotherapy. Median overall survival was 10.7 months in the patients who had received radiation, compared with 5.3 months in patients who were radiation-naive.[14] The results of this study support the potential for synergy between radiotherapy and immunotherapy. It is also interesting to consider that in the KEYNOTE-001 secondary analysis, patients received radiotherapy before they received pembrolizumab, while in the case report described previously, the patient received radiation after treatment with ipilimumab. Emerging evidence for synergy between radiotherapy and immunotherapy has thus focused attention on how best to combine radiotherapy and immunotherapy.
Maximizing Therapeutic Returns: Questions of Timing, Dose, and Fractionation
Timing
There have been no randomized controlled trials to assess the comhere have been no randomized controlled trials to assess the comparative effectiveness of administering a checkpoint inhibitor before vs after radiation. Data in the preclinical setting also are lacking. An argument could be made either way. Use of radiotherapy after checkpoint inhibition is intuitively attractive. Under such circumstances, the adaptive immune system is in a state of readiness to respond to antigen liberated by radiation-induced cell death. In a murine model of metastatic breast cancer, administering a checkpoint inhibitor prior to surgical resection of the tumor resulted in significantly longer survival than giving the drug at the time of surgery.[15] Although this study evaluated sequencing with surgery rather than radiation, the findings may translate to radiotherapy, as tumor antigen is potentially liberated in both settings.
Radiotherapy prior to checkpoint inhibition may allow for more effective immunologic priming-meaning that a strong initial immune response is mounted against a broader array of epitopes as more tumor antigen is liberated in the setting of radiation-induced cell death. Those activated antigen-specific T cells would then be amenable to reversal of exhaustion and positioned to mount an effective antitumoral response in the setting of checkpoint inhibition. In the secondary analysis of the KEYNOTE-001 study discussed above, a relationship was found between prior radiotherapy and pembrolizumab-induced autoimmune toxicity. This implies that in the setting of radiotherapy, not only is priming against tumor antigen improved, but priming of the antiself response may be enhanced as well.
Dose and fractionation
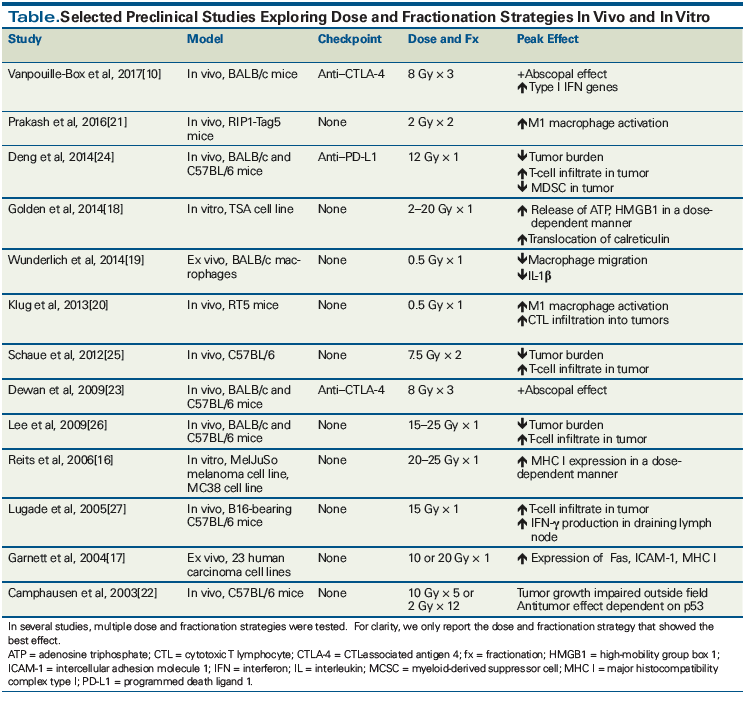
Data in the preclinical setting have been mixed regarding the dose and fractionation strategies that elicit proinflammatory vs anti-inflammatory responses. Multiple in vitro and ex vivo studies of cell lines exposed to varying doses of radiation have shown increased immunologic activation after single doses of 2 to 20 Gy.[16,17] Indicators of immune activation have included increased expression of Fas, ICAM-1 (intercellular adhesion molecule 1), and class I major histocompatibility complex. Release of adenosine triphosphate and HMGB1 (high-mobility group B1), markers of proinflammatory cell death, has also been demonstrated.[18] An ex vivo model examining the effect of low-dose radiation (0.1 to 0.5 Gy) showed that macrophages behave in an anti-inflammatory manner, exhibiting reduced chemotaxis and decreased cytokine production.[19] In the complex context of the tumor microenvironment, however, data collected from cell lines must be interpreted with caution. As a case in point, an in vivo murine model of spontaneous pancreatic islet carcinogenesis was used to study the effect of single doses of radiation of 0.5 to 6 Gy in mice. After 7 days, tumoral T-cell infiltration was assessed and it was found that the lowest radiation dose, 0.5 Gy, resulted in the most infiltrating T cells. This T-cell recruitment required the expression of inducible nitric oxide synthase in tumor-associated macrophages. Tumor burden was reduced and survival increased in mice treated with these low radiation doses.[20,21] The results of this study are at odds with what was seen in the ex vivo model described previously, and the discordance serves as a reminder that immunologic activation following radiation is dependent on the context in which radiation is given.
Case reports describe the abscopal effect in the settings of both standard fractionation and hypofractionation. In the 46 cases described in the literature between 1969 and 2014, doses ranging from 0.45 Gy to 60.75 Gy were used, with a median total dose of 31 Gy and a median dose per fraction of 3 Gy.[12] Preclinical studies also report systemic antitumoral effects with a variety of dose and fractionation strategies. Camphausen et al demonstrated that mice bearing Lewis lung carcinoma or fibrosarcoma tumors showed impaired growth of distant tumors after irradiation of a single tumor on the leg. Two fractionation strategies were used: 24 Gy in 12 fractions and 50 Gy in 5 fractions. They noted a dose-dependent effect, with the 50-Gy dose demonstrating better distant tumor control.[22] Evidence has accumulated that the etiology of the abscopal effect is immunologic in nature, and this has prompted interest in assessing the combination of immunotherapy and radiotherapy in the preclinical setting.
KEY POINTS
- The innate immune system and the adaptive immune system work together to generate systemic immune responses.
- Radiation causes DNA damage that activates the innate immune system, while the death of tumor cells provides tumor antigen for the adaptive immune system to target.
- Hypofractionated radiotherapy may maximize the immunologic antitumoral response, but more basic science and prospective trials are needed.
Murine models have been developed to study the dose and fractionation strategy best able to elicit an abscopal effect in the context of checkpoint inhibition. The strongest effects have been seen with hypofractionated regimens. A study by Dewan et al used mice bearing TSA breast carcinoma tumors on either flank, to interrogate dose and fractionation strategy in the setting of cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) blockade. Three different regimens were used: 20 Gy × 1; 8 Gy × 3; or 6 Gy × 5. Single-dose radiotherapy failed to induce an abscopal response in the presence of anti–CTLA-4, while both fractionated regimens generated abscopal responses.[23] A similar synergy between radiation and checkpoint inhibition was noted in an in vivo murine model described by Deng et al; however, this study used an anti–programmed death ligand 1 (PD-L1) antibody and a dose of 12 Gy in a single fraction to demonstrate improved tumor control and increased T-cell infiltrate compared with either modality alone.[24] The importance of fractionation is also supported by a preclinical study by Schaue et al, which used different fractionation regimens to treat mice bearing B16 melanomas and found that a fractionation regimen of 7.5 Gy × 2 resulted in maximal tumor control and a relatively low number of infiltrating regulatory T cells.[25] Other studies in murine models (detailed more fully in the Table) have used large doses of radiation in a single fraction in the absence of checkpoint inhibition and have shown increased T-cell infiltrates, decreased tumor burden, and increased production of effector cytokines relative to lower doses.[26,27]
Limited clinical studies in the setting of checkpoint inhibition
Case reports detailing the abscopal effect often involve hypofractionated radiation: In the study of the 33-year-old woman with metastatic melanoma discussed previously, Postow et al observed an abscopal effect in the patient, who received 9.5 Gy × 3 fractions[13]; Hiniker et al reported an abscopal response in a melanoma patient who received 17 Gy × 3 fractions[28]; Golden et al described an abscopal response in a patient with lung cancer who had received 6 Gy × 5 to a liver metastasis.[29] There is not yet sufficient evidence to formally recommend a particular dose/fractionation regimen in the clinical setting. Randomized clinical trials of different fractionation regimens in the setting of immunotherapy will be required before the optimal strategy can be identified. Trial designs should take into account the preclinical data that indicate a potential superiority of hypofractionated regimens, and the indications that a single fraction of radiation may be insufficient to promote an optimal antitumoral response.
Choice of target
In addition to considerations of dose and fractionation, it is possible that not all target sites are immunologically equal. Most case reports describing abscopal effects have involved visceral organs rather than bone.[30] Immune privilege refers to the phenomenon of certain sites in the body being more tolerant of antigen than others, and thus less apt to facilitate development of an antitumoral response after radiation. A full discussion of this concept is beyond the scope of this article, but bone marrow has been proposed as a site of immune privilege,[31] and it may be that hypofractionated radiation to bone is less likely to be effective at eliciting an abscopal response than radiation to other target sites. Another outstanding question is whether nodal irradiation is beneficial or detrimental to an effective antitumoral response. Reduction of tumor burden and release of additional antigen resulting from treatment of affected nodes is an obvious benefit of nodal radiation, but prophylactic treatment of unaffected nodes may diminish the adaptive immune system’s capacity for mounting an effective defense.
Conclusions
The immune system’s ability to recognize a tumor as foreign and mount a systemic defense is a powerful therapeutic tool. Significant questions remain before radiotherapy-induced immune activation can prove more reliable than existing treatment modalities. Chief among these are:
• What is the optimal strategy for sequencing radiation therapy and immunotherapy?
• What is the optimal dose and fractionation strategy for maximizing the antitumoral immune response?
• Are all possible target lesions equivalent, or are some sites more conducive to immune activation than others?
Clinical trials that address dose and timing will answer some of these questions,[30] but the importance of additional basic science research to guide trial design cannot be overemphasized. Further investigation into how the interaction between the tumor microenvironment and the host immune system influences response to different dose and fractionation strategies will form the basis for improving available therapeutic options and increasing the applicability and efficacy of radiation in the clinical setting.
Financial Disclosure:Dr. Decker receives research support from Genentech and Merck, and has had consulting agreements with AstraZeneca, Merck, and Regeneron. Dr. Campbell has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Foray N. Victor Despeignes, the forgotten pioneer of radiation oncology. Int J Radiat Oncol Biol Phys. 2016;96:717-21.
2. Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153.
3. Hancock J. Life, death, and apoptosis [chapter 14]. In: Cell Signaling. Oxford University Press; 2010. Page 315.
4. Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353-63.
5. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99-109.
6. Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol. 2017;12:103-30.
7. Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111.
8. Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514-8.
9. Hu B, Jin C, Li HB, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765-8.
10. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618.
11. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862-70.
12. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25-37.
13. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925-31.
14. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895-903.
15. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382-99.
16. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259-71.
17. Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985-94.
18. Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518.
19. Wunderlich R, Ernst A, Rödel F, et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179:50-61.
20. Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589-602.
21. Prakash H, Klug F, Nadella V, et al. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis. 2016;37:301-13.
22. Camphausen K, Moses MA, Ménard C, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990-3.
23. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379-88.
24. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687-95.
25. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306-10.
26. Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589-95.
27. Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516-23.
28. Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5:404-7.
29. Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365-72.
30. Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51.
31. Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216-9.