Hodgkin’s lymphoma
In the year 2005, approximately 8,000 new cases of Hodgkin's lymphoma (HL)will be diagnosed in the United States. Over the past 4 decades, advances inradiation therapy and the advent of combination chemotherapy have tripledthe cure rate of patients with HL. In 2005, more than 80% of all newly diagnosedpatients can expect a normal, disease-free life span.
In the year 2005, approximately 8,000 new cases of Hodgkin's lymphoma (HL)will be diagnosed in the United States. Over the past 4 decades, advances inradiation therapy and the advent of combination chemotherapy have tripledthe cure rate of patients with HL. In 2005, more than 80% of all newly diagnosedpatients can expect a normal, disease-free life span.EpidemiologyGender The male-to-female ratio of HL patients is 1.3:1.0.Age The age-specific incidence of the disease is bimodal, with the greatestpeak in the third decade of life and a second, smaller peak after the age of 50years.Race HL occurs less commonly in African-Americans (2.3 cases per 100,000persons) than in Caucasians (3.0 per 100,000 persons).Geography The age-specific incidence of HL differs markedly in different countries.In Japan, the overall incidence is low and the early peak is absent. Insome developing countries, there is a downward shift of the first peak intochildhood.Etiology and risk factorsThe cause of HL remains unknown, and thereare no well-defined risk factors for its development.However, certain associations havebeen noted that provide clues to possible etiologicfactors.Familial factors For example, same-sex siblingsof patients with HL have a 10 timeshigher risk for the disease. Patient-child combinationsare more common than spouse pairings.Higher risk for HL is associated with fewsiblings, single-family houses, early birthorder, and fewer playmates-all of which decreaseexposure to infectious agents at an earlyage. The monozygotic twin sibling of a patientwith Hodgkin's disease has a 99 timeshigher risk of developing HL than a dizygotictwin sibling of a patient with HL. Theseassociations suggest a genetic predispositionand/or a role for an infectious or environmentalagent during childhood or earlyadolescence in the etiology of the disease.Viruses Familial aggregation may imply geneticfactors, but other epidemiologic findingsmentioned previously suggest an abnormalresponse to an infective agent. Both factorsmay play a role in the pathogenesis of the disease.The Epstein-Barr virus (EBV) has beenimplicated in the etiology of HL by both epidemiologicand serologic studies, as well asby the detection of the EBV genome in 20%-80% of tumor specimens.There have been no conclusive studies regardingthe possible increased frequency of HL inpatients with human immunodeficiency virus(HIV) infection. However, HL in HIV-positivepatients is associated with an advancedstage and poor therapeutic outcome. (For furtherdiscussion of HL in patients with HIV infection,see chapter 27.)Signs and symptomsHL is a lymph node-based malignancy andcommonly presents as an asymptomaticlymphadenopathy that may progress to predictableclinical sites.Location of lymphadenopathy More than80% of patients with HL present with lymphadenopathyabove the diaphragm, often involvingthe anterior mediastinum; the spleenmay be involved in about 30% of patients. Lessthan 10%-20% of patients present with lymphadenopathylimited to regions below the diaphragm.The commonly involved peripherallymph nodes are located in the cervical, supraclavicular,and axillary areas; para-aorticpelvic and inguinal areas are involved less frequently.Disseminated lymphadenopathy israre in patients with HL, as is involvement ofWaldeyer's ring and occipital, epitrochlear,posterior mediastinal, and mesenteric sites.Systemic symptoms About 30% of patientsexperience systemic symptoms. They includefever, night sweats, or weight loss (so-called Bsymptoms) and chronic pruritus. These symptomsoccur more frequently in older patientsand have a negative impact on prognosis (seesection on "Staging and prognosis").Extranodal involvement HL may affectextranodal tissues by direct invasion (contiguity;the so-called E lesion) or by hematogenousdissemination (stage IV disease). Themost commonly involved extranodal site isthe lungs. Liver, bone marrow, and bone mayalso be involved.DiagnosisThe initial diagnosis of HL can only be madeby biopsy. Because reactive hyperplasticnodes may be present, multiple biopsies of asuspicious site may be necessary. Needle aspirationis inadequate because the architectureof the lymph node is important for diagnosisandhistologicsubclassification.PathologyReed-Sternberg cell
In a biopsied lymph node, the Reed-Sternberg(R-S) cell is the diagnostic tumor cell that mustbe identified within the appropriate cellularmilieu of lymphocytes, eosinophils, and histiocytes.HL is a unique malignancy pathologicallyin that the tumor cells constitute aminority of the cell population, whereas normalinflammatory cells are the major cell component.As a result, it may be difficult to identifyR-S cells in some specimens. Also, otherlymphoproliferations may have cells resemblingR-S cells.The R-S cell is characterized by its large sizeand classic binucleated structure with large eosi-nophilic nucleoli. Two antigenic markers are thought to provide diagnostic information:CD30 (Ber-H2) and CD15 (Leu-M1). These markers are present on R-Scells and their variants but not on background inflammatory cells.Recent studies have confirmed the B-cell origin of the R-S cell. Single-cell polymerasechain reaction (PCR) analysis of classic R-S cells shows a follicular centerB-cell origin for these cells with clonally rearranged but crippled V heavychaingenes, presumably leading to inhibition of apoptosis. Also, high levels ofthe nuclear transcription factor-kappa-B (NF-κB) have been found in R-S cells;these high NF-κB levels may play a role in pathogenesis by interfering withapoptosis.Histologic subtypes
According to the Rye classification (based on the number and appearance ofR-S cells, as well as the background cellular milieu), there are four histologicsubtypes of HL.Nodular sclerosis, the most common subtype, is typically seen in young adults(more commonly in females) who have early-stage supradiaphragmatic presentations.Its distinct features are the presence of (1) broad birefringent bands ofcollagen that divide the lymphoid tissue into macroscopic nodules and (2) anR-S cell variant, the lacunar cell.Mixed cellularity is the second most common histology. It is more often diagnosedin males, who usually present with generalized lymphadenopathy orextranodal disease and with associated systemic symptoms. R-S cells are frequentlyidentified; bands of collagen are absent, although a fine reticular fibrosismay be present; and the cellular background includes lymphocytes, eosinophils,neutrophils, and histiocytes.Lymphocyte-predominant HL is an infrequent form of HL in which few R-Scells or their variants may be identified. The cellular background consists primarilyof lymphocytes in a nodular or sometimes diffuse pattern. The R-S variantsexpress a B-cell phenotype (CD20-positive, CD15-negative). B-cell clonalityhas also been demonstrated by PCR of the immunoglobulin heavy-chain genesin single R-S variant cells in biopsy material from patients with lymphocytepredominantHL.This finding has led investigators to propose that lymphocyte-predominant HLis a B-cell malignancy with a mature B-cell phenotype, distinct from the otherthree histologic types of HL. Lymphocyte-predominant HL is often clinicallylocalized, is usually treated effectively with irradiation alone, and may relapselate (a clinical feature reminiscent of low-grade lymphoma). The 15-year disease-specific survival is excellent (> 90%).The World Health Organization (WHO) classification recognizes a new subtypeof lymphocyte-rich classic HL that has morphologic similarity to nodularlymphocyte-predominant HL. However, the R-S cells have a classic morphologyand phenotype (CD30-positive, CD15-positive, CD20-negative), and thesurrounding lymphocytes are reactive T cells. This disease subtype does notshow a tendency for late relapse and should be managed like other classicHodgkin's lymphoma histologies.Lymphocyte depletion is a rarediagnosis, particularly since theadvent of antigen marker studies,which led to the recognitionthat many such cases representedT-cell non-Hodgkin's lymphomas(NHLs). R-S cells are numerous,the cellular background issparse, and there may be diffusefibrosis and necrosis. Patientsusually have advanced-stage disease,extranodal involvement, anaggressive clinical course, and apoor prognosis.
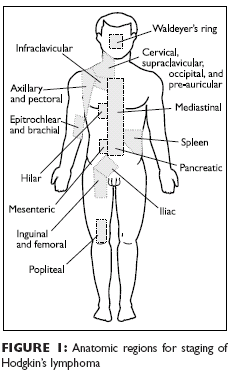
Staging andprognosisPrecise definition of the extentof nodal and extranodal involvementwith HL according toa standard staging classificationsystem is critical for selection ofthe proper treatment strategy.Staging system
The staging system is detailed in Table 1, and the anatomic regions that providethe basis for the staging classification are illustrated in Figure 1. The assignmentof stage is based on:
- the number of involved sites
- whether lymph nodes are involved on both sides of the diaphragm andwhether this involvement is bulky (particularly in the mediastinum)
- whether there is contiguous extranodal involvement (E sites) or disseminatedextranodal disease
- whether typical systemic symptoms (B symptoms) are present.
In defining the disease stage, it is important to note how the information wasobtained, since this fact reflects on remaining uncertainties in the evaluationfor extent of disease. Clinical staging refers to information that has been obtainedby initial biopsy, history, physical examination, and laboratory and radiographicstudies only. A pathologic stage is determined by more extensive surgicalassessment of potentially involved sites, eg, by surgical staging laparotomyand splenectomy.
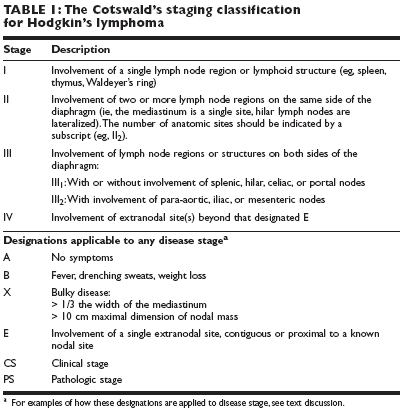
Also, various designations relating to the presence or absence of B symptomsor bulky disease (see Table 1) can be applied to any disease stage. For example,a patient with no B symptoms but with a bulky mediastinal mass and involvementof the cervical lymph nodes would be defined as having CS IIAX disease.A patient with axillary disease and fever who underwent a staging laparotomythat revealed involvement of the para-aortic lymph nodes and spleenwould be staged as PS III
2
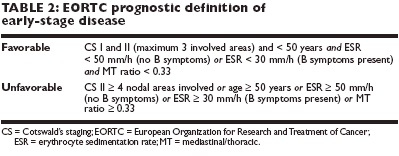
B.Most recent studies in stage I/II distinguish between favorable and unfavorableearly-stage disease, according to the European Organization for Researchand Treatment of Cancer (EORTC) definitions outlined in Table 2.
Clinical staging evaluation
Disease-associated symptoms
As mentioned previously, disease-associatedsymptoms may occur in up to one-third of patients. They may include Bsymptoms, pruritus, and, less commonly, pain in involved regions after ingestionof alcohol. In each anatomic stage, the presence of B symptoms is an adverseprognostic indicator and may strongly affect treatment choices. B symp
toms are carefully defined in the staging system. Unexplained fever should be> 38
o
C and recurrent during the previous month, night sweats should be drenchingand recurrent, and unexplained weight loss is significant only if > 10% ofbody weight has been lost within the preceding 6 months. Although pruritus isno longer considered to be a B symptom, the presence of generalized itchingmay be considered to be an adverse prognostic symptom.Certain combinations of B symptoms are more prognostically significant thanothers. For example, the combination of fever and weight loss has a worseprognosis than do night sweats alone.
Physical examination
should carefully determine the location and size of allpalpable lymph nodes. Inspection of Waldeyer's ring, detection of splenomegalyor hepatomegaly, and evaluation of cardiac and respiratory status areimportant.
Laboratory studies
should include a CBC with WBC differential and plateletcount, the erythrocyte sedimentation rate (ESR), tests for liver and renal function,and assays for serum alkaline phosphatase and lactate dehydrogenase(LDH). A moderate to marked leukemoid reaction and thrombocytosis arecommon, particularly in symptomatic patients, and usually disappear withtreatment.
ESR
The ESR may provide helpful prognostic information. At some centers,treatment programs for patients with early-stage disease are influenced by thedegree of ESR elevation. In addition, changes in the ESR following therapymay correlate with response and relapse.
Abnormalities of liver function
studies should prompt further evaluation of thatorgan, with imaging and possible biopsy.
Alkaline phosphatase
An elevated alkaline phosphatase level may be a nonspecificmarker, but it may also indicate bone involvement that should be appropriatelyevaluated by a radionuclide bone scan and directed skeletal radiographs.
Imaging studies
Radiologic studies should include a chest x-ray and CT scanof the chest, abdomen, and pelvis with IV contrast. In most patients, PETscan will provide important information on the extent of disease, and a baselinefor evaluation of response to treatment is highly recommended. Radionuclide
bone scan, MRI of the chest or abdomen, and CT scan of the neck are contributoryonly under special circumstances.
Evaluation for supradiaphragmatic disease
The thoracic CT scan details the statusof intrathoracic lymph node groups, the lung parenchyma, pericardium,pleura, and chest wall. Since the chest CT scan may remain abnormal for along time after the completion of therapy, the evaluation of pretreatmentinvolvement and response to therapy is assisted by the use of a PETor gallium scan.
18
F-fluorodeoxyglucose (FDG)-PET scanning provides moreinformation and better resolution than does a gallium scan.
Evaluation of the abdomen and pelvis
A CT scan and PET are the basic imagingstudies for evaluation of the abdomen and pelvis.
Bone marrow biopsy
Bone marrow involvement is relatively uncommon,but because of the impact of a positive biopsy on further staging and treatment,unilateral bone marrow biopsy should be part of the staging process of patientswith stage IIB disease or higher.
Lymphangiography and staging laparotomy
These two old methods of staging have been replaced by modern imagingtechniques using high-resolution CT scanning and FDG-PET.
Treatment
HL is sensitive to radiation and many chemotherapeutic drugs, and, in moststages, there is more than one effective treatment option. Disease stage is themost important determinant of treatment options and outcome. All patients,regardless of stage, can and should be treated with curative intent.
TREATMENT OF STAGE I/II DISEASE
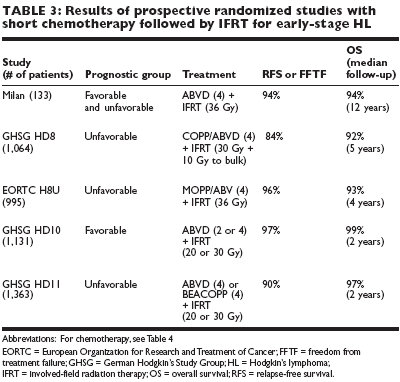
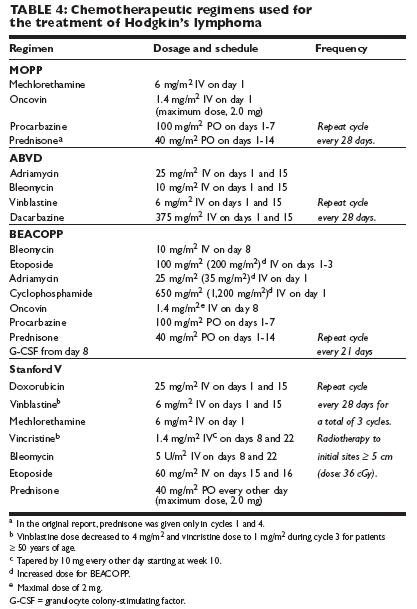
The treatment of choice for favorable and unfavorable early-stage classic HL isbrief chemotherapy followed by involved-field radiotherapy (IFRT). Most ofthe experience that yielded excellent treatment results with low toxicity waswith ABVD (Adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine[DTIC-Dome]) 4 and IFRT of 30 to 36 Gy. Table 3 summarizes data fromrandomized studies that reported on the combination of short chemotherapy(four or even only two cycles) followed by IFRT. The three top randomizedstudies have also indicated that adding extended-field radiotherapy to chemotherapyis not necessary and the small involved field is adequate.The most recent (and as yet fully mature) excellent results are with shorteningthe duration of chemotherapy to only two cycles of ABVD in favorable patientsand reducing the IFRT dose to 20 Gy (Table 3). If the excellent resultsobtained by the German Hodgkin's Study Group prevail with additional follow-up, brief ABVD and low-dose IFRT will become the standard of care forfavorable early-stage HL.
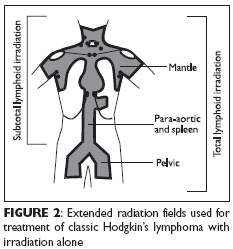
Subtotal lymphoid irradiation
(ie, treatment of the mantle and para-aortic fieldsonly) remains an adequate alternative treatment of clinically or pathologically stagedfavorable (nonbulky and without B symptoms) early-stage HL (stage I/II). Yet thisoption is no longer the treatment of choice due to the risk of second tumors and(to a lesser degree) coronary artery disease in long-term survivors of extensiveradiotherapy alone as practiced in the past. In classic (non-lymphocyte predominant)HL, subtotal lymphoid irradiation is adequate for patients who are not candidatesfor a chemotherapy-containing strategy.In patients who underwent pathologic staging (laparotomy) and were treatedwith primary irradiation alone, several large series reported a 15- to 20-year survivalrate of nearly 90% and a relapse-free survival rate of 75%-80%. Most relapses(75%) occurred within 3 years after the completion of therapy; late relapseswere uncommon. More than half of the patients who relapsed after radiotherapyalone were still curable with standard chemotherapy.Canadian and European studies have reported excellent overall survival results inpatients selected for radiotherapy on the basis of clinical prognostic factors alone. Thus,irradiation alone can be safely offered to clinically staged patients with favorable prognosticfactors who are not candidates for combined-modality treatment.
Chemotherapy alone
In two prospective, randomized studies, radiotherapyalone was as effective as or superior to MOPP (mechlorethamine [Mustargen],Oncovin [vincristine], procarbazine [Matulane], and prednisone) chemotherapyin improving the survival of patients with early-stage disease. Although the relapserate after chemotherapy is similar to that after radiotherapy, conventionaldosesalvage chemotherapy used after failure of chemotherapy has poor results,which translates into an inferior overall survival.More recently, five prospective randomized studies compared chemotherapyalone with chemotherapy followed by IFRT or regional radiotherapy in patientswith early-stage HL. The Children's Cancer Group (CCG) tested therole of radiation therapy in young patients (< 21 years old) who attained acomplete response with risk-adapted chemotherapy (mostly COPP [cyclophosphamide(Cytoxan, Neosar), Oncovin (vincristine), procarbazine, and prednisone)/ABV [Adriamycin (doxorubicin), bleomycin, and vinblastine], four tosix cycles). They enrolled 829 patients into the study (68% had early-stage disease);501 patients who achieved a complete response were then randomizedto receive either low-dose (21 Gy) IFRT or no further treatment. The accrualwas stopped earlier than planned because of a significantly higher number ofrelapses on the no-radiotherapy arm. The 3-year event-free survival with anintent-to-treat analysis was 92% for patients randomized to receive radiotherapyand 87% for those randomized to receive no further treatment (
P
= .057).The EORTC/Groupe d'Etude des lymphomes de l'adulte (GELA) conducteda large randomized trial in patients with favorable early-stage classic HL. Allpatients received six cycles of EBVP (epirubicin [Ellence], bleomycin, vinblastine,and prednisone). Only patients who achieved a complete response arerandomized to receive either IFRT of 36 Gy, IFRT of 20 Gy, or no irradiation.After an interim analysis, the EORTC/GELA groups closed the entry to theno-radiotherapy arm because of the excessive number of relapses.The National Cancer Institute of Canada and the Eastern Cooperative OncologyGroup (ECOG) included 405 patients with nonbulky stage I/II disease.They were randomized to receive either "standard therapy," namely, subtotalnodal irradiation (STNI) for favorable patients, and ABVD (two cycles) followedby STNI for unfavorable (B, elevated ESR, ≥ 3 sites, age ≥ 40, mixedcellularity histology) patients or experimental therapy consisting of six cyclesor four cycles (if complete response was attained after two cycles) of ABVDand no radiotherapy. At a median follow-up of 4.2 years, progression-free survivalwith ABVD alone was significantly inferior (
P
= .006; hazard ratio = 2.6;5-year estimates of disease progression-free survival, 87% vs 93%). At this earlypoint, no survival difference has been detected.The Memorial Sloan-Kettering Cancer Center trial included 152 patients withnonbulky early-stage HL. Patients were randomized up front to receive eitherABVD 6 alone or ABVD 6 followed by radiotherapy. At 60 months, theduration of complete response and freedom from disease progression for ABVDand radiotherapy vs ABVD alone are 91% vs 87% (
P
= .61) and 86% vs 81%(
P
= .61), respectively. Overall survival was 97% with ABVD and radiotherapyvs 90% with ABVD alone (
P
= .08). Although the differences between the outcome of the two treatment groups were not statistically significant, the studywas not powered to detect differences between the treatment strategies thatwere smaller than 20%, due to the small number of patients and events.In a prospectively randomized study reported from India, patients with HLwho achieved a complete response after ABVD were randomized to receiveeither IFRT or no further therapy. The 8-year event-free survival and overallsurvival were significantly better for the patients who received consolidationwith IFRT than for those who received ABVD alone. Subset analysis indicatedthat the benefit from added IFRT was more prominent in advanced stage thanin early stage.The National Comprehensive Cancer Network (NCCN) guidelines recommendcombined-modality therapy as the treatment of choice for favorable or unfavorableclassic HL. Opinions differ as to the use of chemotherapy alone and itremains highly controversial.
TECHNICAL ASPECTS OF RADIATION THERAPY
Involved-field radiotherapy
In a combined-modality setting, irradiation of the involved lymph node chain,with or without adjacent sites, and tailoring of the field borders to thepostchemotherapy tumor volume (in critical areas such as the mediastinum)are recommended. IFRT is the most appropriate irradiation approach afterchemotherapy. IFRT or regional radiotherapy alone is used for lymphocytepredominantHL. Recommendations for IFRT for HL are detailed in an articleby Yahalom and Mauch.
Extended radiation fields
Successful therapy with irradiation alone in classic HL requires treatment of allclinically involved lymph nodes and all nodal and extranodal regions at riskfor subclinical involvement (Figure 2). The HL radiation fields were designedto conform to the philosophy of treating regions beyond the immediately involvedarea while accounting for normal tissue tolerance and the technical constraintsof field size. The extended radiation fields are inappropriate when radiationis administered as consolidation following chemotherapy and thus arerarely used today.
Dose considerations
When irradiation alone is used to treat HL, the standard total dose to eachfield is 3,600 cGy, delivered in daily fractions of 180 cGy over 4 weeks. Inaddition, clinically involved areas are given a boost of 360-540 cGy in 2-3fractions to bring the total dose to these areas up to 3,960-4,140 cGy. Patientswho receive irradiation as consolidation after chemotherapy receive a total doseof 2,000-3,600 cGy in 150-180-cGy fractions. We normally use opposed anteriorand posterior fields that are evenly weighted and treat both fields daily.Three-dimensional conformal radiotherapy and intensity-modulated radiationtherapy (IMRT) are employed for selected cases.
SIDE EFFECTS ANDCOMPLICATIONS OFRADIOTHERAPY
Side effects of radiotherapy dependon the irradiated volume,dose administered, and techniqueemployed. They are alsoinfluenced by the extent andtype of prior chemotherapy, ifany, and by the patient's age.
Acute effects
The potentialacute side effects of involvedfields in the upper body includemouth dryness, change in taste,pharyngitis, nausea, dry cough,dermatitis, and fatigue. Theseside effects are usually mild andtransient.
The main potential side effects of subdiaphragmatic irradiation are loss of appetite,nausea, and increased bowel movements.These reactions are usually mild andcan be minimized with standard antiemeticmedications.Irradiation of more than one field, particularlyafter chemotherapy, can cause myelosuppression,which may necessitate treatment delays.
Delayed effects
Delayed side effects maydevelop anywhere from several weeks to severalyears after the completion of radiotherapy.
Lhermitte's sign
Approximately 15% of patientswho receive the full dose of radiation to theneck may note an electric shock sensation radiatingdown the backs of both legs when thehead is flexed (Lhermitte's sign) 6 weeks to 3months after mantle-field radiotherapy. Possiblysecondary to transient demyelinizationof the spinal cord, Lhermitte's sign resolvesspontaneously after a few months and is notassociated with late or permanent spinal corddamage.
Pneumonitis and pericarditis
During the sameperiod, radiation pneumonitis and/or acutepericarditis may occur in < 5% of patients whoreceive large fields of radiation to the medi-astinum; these side effects occur more often in those who have extensive mediastinaldisease. Both inflammatory processes have become rare with modernradiation techniques.
Herpes zoster infection
Patients with HL, regardless of treatment type, have apropensity to develop herpes zoster infection within 2 years after therapy. Usually,the infection is confined to a single dermatome and is self-limited. If thecutaneous eruption is identified promptly, treatment with systemic acyclovirwill limit its duration and intensity.
Subclinical hypothyroidism
Radiotherapy of the neck can induce subclinicalhypothyroidism in about one-third of patients. This condition is detected byelevation of thyroid-stimulating hormone (TSH). Thyroid replacement withlevothyroxine (T
4
) is recommended, even in asymptomatic patients, to preventovert hypothyroidism and decrease the risk of benign thyroid nodules.
Infertility
Irradiation of the pelvis may have deleterious effects on fertility. Inmost patients, this problem can be avoided by appropriate gonadal shielding. Infemales, the ovaries can be moved into a shielded area laterally or inferomediallynear the uterine cervix. Irradiation fields that spare the pelvis do not increase therisk of sterility.
Secondary malignancies
Patients with HL who were cured with radiotherapyand/or chemotherapy have an increased risk of secondary solid tumors (mostcommonly, lung, breast, and stomach cancers, as well as melanoma) and NHL10 or more years after treatment. Chemotherapy combinations that do notinclude alkylating agents or a new brief program (such as Stanford V) as well asthe smaller involved fields and lower doses are less likely to have the increasedrisk observed with extended fields and/or MOPP or MOPP-like chemotherapy.
Lung cancer
Patients who are smokers should be strongly encouraged to quit thehabit because the increase in lung cancer that occurs after irradiation orchemotherapy with alkylating agents has been detected mostly in smokers.Alkylating agents (such as in the MOPP regimen) and radiation therapy wereassociated with an increased risk of lung cancer in an additive and dosedependentfashion. These effects were multiplied by tobacco use (see SuggestedReading).
Breast cancer
The increase in breast cancer risk is inversely related to the patient'sage at HL treatment; no increased risk has been found in women irradiatedafter 30 years of age. The risk of breast cancer is increased with higher radiationbreast dose and is reduced in patients who received chemotherapy or ovarianirradiation that induced early menopause. In a case-controlled study performedat The Netherlands Cancer Institute, 48 women with breast cancerafter HL were matched with 175 women with HL and no breast cancer. Thestudy demonstrated that women who received chemotherapy followed by radiationtherapy had a significantly decreased relative risk (RR) of developingbreast cancer as compared with patients treated with irradiation alone(RR = 0.45;
P
= .005). For patients treated with irradiation alone, the risk ofbreast cancer increased significantly with increasing radiation dose to the breast(
P
trend = .01). Reaching menopause before the age of 35 years after treatment
with chemotherapy and irradiation was associatedwith a markedly reduced risk of breastcancer (RR = 0.06;
P
= .001). The risk reductionwith combined-modality treatment indicatesthe importance of ovarian function inthe tumorigenesis process of radiation-inducedbreast cancer. A larger international case-controlledstudy of 105 breast cancer patients and266 controls had similar findings.In most situations, modern involved-field radiotherapyshould spare the breast. Breast canceris curable in its early stages, and earlydetection has a significant impact on survival.Breast examination should be part of the routinefollow-up for women cured of HL, androutine mammography should begin about 8years after treatment.
Cardiovascular disease
An increased risk ofcardiovascular morbidity has been reportedamong patients who have received mediastinalirradiation. In a retrospective study evaluating the cardiac risks of 450 patientscured of HL with radiotherapy aloneor in combination, 42 patients (10%) developedcoronary artery disease (CAD) at a medianof 9 years after treatment, 30 patients (7%)developed carotid and/or subclavian arterydisease at a median of 17 years after treatment,and 25 patients (6%) developed clinically significantvalvular dysfunction at a median of22 years. The most common valve lesion wasaortic stenosis, which occurred in 14 valves.The only treatment-related factor associatedwith the development of CAD was use of aradiation technique that resulted in a highertotal dose to a portion of the heart (RR = 7.8;95% CI = 1.1-53.2;
P
=.04). No specific treatment-related factor was associated with carotidor subclavian artery disease or valvular dysfunction.Freedom from any cardiovascularmorbidity was 88% at 15 years and 84% at 20years.To reduce this hazard, radiation fields shouldconform to the involved postchemotherapyvolume, and the dose should be reduced to 20-30 Gy if possible. Patients who have receivedradiation to the mediastinum should be moni-
tored and advised about other established coronary disease risk factors, such assmoking, hyperlipidemia, hypertension, and poor dietary and exercise habits.Cholesterol levels should be monitored and treated if elevated.
Effects on bone and muscle growth
In children, high-dose irradiation willaffect bone and muscle growth and may result in deformities. Current treatmentprograms for pediatric HL are chemotherapy-based; radiotherapy is limitedto low doses.
TREATMENT OF STAGE III/IV DISEASE
Chemotherapy has become curative for many patients with advanced stages ofHL. MOPP has been the primary effective combination chemotherapy regimenfor advanced-stage disease since the 1960s. Over the past several years, ABVD hasbeen shown to be more effective and less toxic than MOPP, particularly with respectto sterility and secondary leukemia.
Combination chemotherapy regimens
Doxorubicin-containing regimens
A doxorubicin-containing regimen, suchas ABVD (Table 4), is the treatment of choice for patients presenting with stageIII or IV disease, as demonstrated by a randomized phase III trial undertakenby the Cancer and Leukemia Group B (CALGB). This trial showed highercomplete response rates with ABVD and ABVD/MOPP (82% and 83%, respectively)than with MOPP alone (65%).One reason for the improved response ratein the groups treated with doxorubicin-containingregimens was the higher percentageof patients who were able to receive ≥ 85%of the expected chemotherapy dose, particularlyin the ABVD group. In addition, ratesof significant and life-threatening neutropeniawere higher in patients treated with theMOPP-containing regimens than in thosetreated with other regimens.Subsequent trials compared ABVD, alternatingMOPP/ABVD, and a MOPP/ABV hybrid.Alternating MOPP/ABVD and theMOPP/ABV hybrid were found to beequally effective in treating advanced-stageHL. However, a recent intergroup study thatcompared ABVD with MOPP/ABV hybrid(without irradiation) was closed early becauseof concerns of excess treatment-related deathsand second malignancies (mostly acute myelogenousleukemia and lung cancer) in theMOPP/ABV hybrid arm.
Shortened dose-intense regimens
Recently,shortened dose-intense regimens haveshown promise. For example, the 12-weekStanford V regimen (see Table 4) combined with IFRT produced a 5-yearoverall survival rate of 96% and a freedom from-disease-progression rate of 89%. Thefreedom-from-disease-progression rate wassignificantly superior among patients witha prognostic score of 0-2, compared withthose with a score of 3 and higher (94% vs75%;
P
= .0001). Of interest, in 142 patientsfrom Stanford, no secondary leukemia wasobserved, and 42 pregnancies were reported.Another new regimen called BEACOPP(bleomycin, etoposide, Adriamycin [doxorubicin],cyclophosphamide, Oncovin [vincristine],procarbazine, and prednisone)combined with IFRT (in most patients)showed better results than did COPP/ABVD in studies by the German Hodgkin'sLymphoma Study Group.
Combined-modality therapy
Although the role of consolidation radiotherapy after induction chemotherapyremains controversial, irradiation is routinely added in patients with advancedstagedisease who present with bulky disease or who remain in uncertain completeremission after chemotherapy. Retrospective studies have demonstratedthat adding low-dose radiotherapy to all initial disease sites after chemotherapyinducedcomplete response decreases the relapse rate by ~25% and significantlyimproves overall survival.Interpretation of the impact of irradiation in prospective studies has been controversial.However, a Southwest Oncology Group (SWOG) randomized studyof 278 patients with stage III or IV HL suggested that the addition of low-doseirradiation to all sites of initial disease after a complete response to MOP-BAP(mechlorethamine, Oncovin [vincristine], prednisone, bleomycin, Adriamycin[doxorubicin], and procarbazine) chemotherapy improves remission duration inpatients with advanced-stage disease. An intention-to-treat analysis showed thatthe advantage of combined-modality therapy was limited to patients with nodularsclerosis. No survival differences were observed.A recent meta-analysis demonstrated that the addition of radiotherapy to chemotherapyreduces the rate of relapse but did not show a survival benefit forthe combined-modality approach.
LONG-TERM TOXICITIES OFCOMBINATION CHEMOTHERAPY
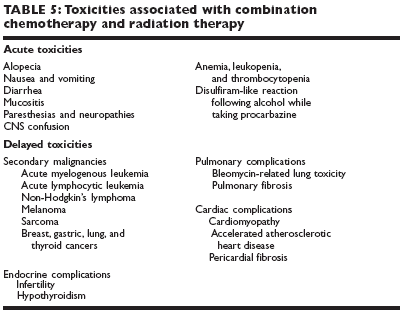
The CALGB trial and the intergroup trials mentioned previously (see sectionon "Combination chemotherapy regimens") noted differences in the long-termtoxicities of various combination chemotherapeutic regimens (Table 5).
Myelodysplasia and acute leukemia
MOPP therapy is known to be related tothe development of myelodysplastic syndromes (MDS) and acute leukemia. Thesesecondary hematologic malignancies begin 2 years following therapy and declineby 10 years, with the maximum risk between 5 and 9 years. Patients withthese malignancies have a poor prognosis.The incidence of secondary leukemia appears to increase with cumulative dosesof chemotherapy, age > 40 years when receiving chemotherapy for HL, andsplenectomy. It is controversial whether combined-modality therapy increasesthe risk of leukemia compared with chemotherapy alone.Cytogenetic studies of secondary leukemias reveal a loss of the long arm ofchromosome 5 and/or 7. Less frequently, there is a loss of chromosome 18 orrearrangement of the short arm of chromosome 17. A balanced rearrangementof 11q23 and 2lq22 also has been described with etoposide therapy.
Other malignancies
also are being observed with increasing frequency afterchemotherapy (most regimens included alkylating agents), particularly lungcancer and NHL. These malignancies have a longer latency period and usuallyare not observed until 15 years after therapy.
Infertility
is another long-term complication seenwith combination chemotherapy. At least 80%of males are found to have permanent azoospermiaor oligospermia following more than threecycles of MOPP chemotherapy; < 10% of menwill have recovery of spermatogenesis within 1-7 years following the end of chemotherapy. Therisk of infertility with ABVD chemotherapy issignificantly lower than that with MOPP chemotherapy,approximately 15%-25%. All menwho desire childbearing potential followingtherapy should be counseled regarding spermbanking.In females, there is a 50% rate of primary ovarianfailure overall. The risk is 25%-30% in patientstreated at age 25 or younger but increasesto 80%-100% in women older than age25. Many women who do maintain ovarianfunction during chemotherapy will have prematuremenopause following therapy.
Pulmonary complications
have been reportedwith ABVD chemotherapy and are relatedto bleomycin-induced lung toxicity. In aMemorial Sloan-Kettering Cancer Centerstudy of 60 patients with early-stage HL receivingABVD chemotherapy with or withoutmediastinal irradiation, 53% reported dyspneaon exertion or cough during ABVD chemotherapyand 37% had a significant decline in pulmonary function.Bleomycin was discontinued in 23% of patients. Following ABVD therapy,there was a significant decline in median forced vital capacity (FVC) anddiffusing capacity of the lung for carbon monoxide (DLCO). Radiotherapyfollowing ABVD chemotherapy resulted in a further decrease in FVC but didnot significantly affect functional status. At longer follow-up, only 1 of 60patients reported persistent dyspnea on minimal exertion.In the CALGB trial, there were 3 fatal pulmonary complications in 238 patients;all 3 patients were older than age 40.Pulmonary fibrosis has also been described after combined-modality therapy.Pulmonary function testing usually reveals a decreased diffusion capacity andrestrictive changes prior to the onset of symptoms.
Cardiomyopathy
is a recognized complication of doxorubicin therapy but isnot commonly seen in patients receiving ABVD chemotherapy. Patients who aretreated with six cycles of ABVD chemotherapy receive a total doxorubicin doseof 300 mg/m
2
; cardiac toxicity is rarely seen in patients who receive a total dose≤400 mg/m
2
.
MANAGEMENT OF RELAPSED DISEASE
Relapse after radiation therapy
Patients with early-stage HL who relapse afterinitial therapy with irradiation alone haveexcellent complete remission rates and 50%-80% long-term survival rates when treated withMOPP or ABVD. The dose regimens usedfor salvage therapy are the same as those outlinedin Table 4.
Relapse after combinationchemotherapy
Among patients with advanced-stage HL,70%-90% will have complete response totreatment; however, up to one-third of patientswith stage III or IV disease will relapse,usually within the first 3 years after therapy.Various studies have identified the followingpoor prognostic factors for response tofirst-line chemotherapy: B symptoms, age> 45 years, bulky mediastinal disease, extranodalinvolvement, low hematocrit, highESR, high levels of CD30, and high levels of serum interleukin-10 and solubleIL-2 receptor.An International Prognostic Index (IPI) has been devised for advanced HL basedon a retrospective analysis of 1,618 patients from 25 centers. In the final model,seven factors were used: albumin < 4 g/dL, hemoglobin < 10.5 g/dL, male gender,stage IV disease, age ≥ 45 years, WBC ≥ 15,000/L, and lymphocytes < 600/L(or 8% of the WBC count). The worst prognostic group (7%) had a 5-year overallsurvival rate of 56% and a failure-free survival rate of 42%.In a comparison of seven well-known prognostic models for HL applied retrospectivelyto a population of patients with advanced-stage disease, three werefound to be the most predictive of outcome. One was the IPI, mentioned previously.The other two were the Memorial Sloan-Kettering Cancer Center model(employing age, LDH, hematocrit, inguinal nodal involvement, and mediastinalmass bulk) and the Database on Hodgkin's Disease model (employing stage,age, B symptoms, albumin, and gender). Integration of the three models in alinear model improved their predictive power.
High-dose therapy with autologous stem-cell transplantation
The preferredsalvage method for patients who relapsed after combined-modalitytherapy or chemotherapy alone or remained refractory to those programs ishigh-dose chemoradiotherapy with autologous stem-cell transplantation (ASCT).Two randomized studies (from Great Britain and Germany) demonstrated anevent-free survival advantage with the high-dose therapy approach. Althougha significant survival advantage was not observed due to the crossover designof the studies, most patients with refractory disease or postchemotherapy relapseare currently managed with high-dose chemoradiation therapy and ASCT.No standard conditioning regimen has been used in this setting, as patientshave had prior treatment with a variety of combinations of chemotherapyand radiation therapy. Although most patients who have received bone marrowhave been treated with several regimens or have had poorly responsivedisease from initial diagnosis, the complete response rate has ranged from 50%-80%, with approximately 40%-80% of responding patients achieving durableremission.Recent analysis of prognostic factors in patients receiving high-dose salvagetherapy indicated that B symptoms at relapse, extranodal disease, and short(< 1 year) remission or no remission are factors associated with a poor outcome.A recent study from Memorial Sloan-Kettering Cancer Center reported theresults of high-dose chemotherapy with ASCT in 65 patients with relapsed orrefractory HL. At a median follow-up of 43 months, overall survival was estimatedto be 73% and event-free survival was estimated to be 58% by intent-totreatanalysis. In a multivariate logistic regression model, there were three adverseprognostic factors: extranodal sites of relapse or refractory disease, completeremission duration of less than 1 year or refractory disease, and B symptoms.Patients with no or one adverse factor had an overall survival of 90% andan event-free survival of 83%. Patients with two adverse factors had an overallsurvival of 57% and an event-free survival of 27%; those with three adversefactors had an overall survival of 25% and an event-free survival of 10%. Afollow-up study of a risk-adapted approach based on the study previously describedsuggested that patients with adverse prognostic factors may benefit fromfurther augmentation of high-dose programs, including a "double-transplant"for selected patients.
References:
Bonadonna G, Bonfante V, Viviani S, et al:
ABVD plus subtotal nodal versus involvedfieldradiotherapy in early-stage Hodgkin’s disease: Long-term results. J Clin Oncol221:2835-2841, 2004.
Diehl V, Thomas RK, Re D:
Hodgkin's lymphoma: Diagnosis and treatment. LancetOncol 5:19-26, 2004.
Dores GM, Metayer C, Curtis RE, et al:
Second malignant neoplasms among longtermsurvivors of Hodgkin's disease: A population-based evaluation over 25 years. JClin Oncol 20:3484â3494, 2002.
Hoppe RT, Abrams RA, Alam AR, et al:
The NCCN Hodgkin's disease clinical practiceguidelines in oncology, version 1.2004. copyright National Comprehensive CancerNetwork, Inc. Available at: http://www.nccn.org. Accessed 2005.
Horning SJ, Hoppe RT, Breslin S, et al:
Stanford V and radiotherapy for locally extensiveand advanced Hodgkin’s disease: Mature results of a prospective clinical trial. J ClinOncol 20:630â637, 2002.
Hutchings M, Eigtved A, Spect L, et al:
FDG-PET in the clinical management ofHodgkin lymphoma. Crit Rev Oncol Hematol 52:19-32, 2004.
Schmitz N, Pfistner B, Sextro M, et al:
Aggressive conventional chemotherapy comparedwith high-dose chemotherapy with autologous haemopoietic stem-cell transplantationfor relapsed chemosensitive Hodgkin’s disease: A randomized trial. Lancet359:2065â2071, 2002.
Thomas RK, Re D, Wolf J, et al:
Hodgkin's lymphoma: Molecular biology of Hodgkinand Reed-Sternberg cells. Lancet Oncol 5:11-18, 2004.Travis LB, Gospodarowicz M, Curtis RE, et al: Lung cancer following chemotherapyand radiotherapy for Hodgkin's disease. J Natl Cancer Inst 94:182-192, 2002.
Yahalom J, Mauch P:
The involved field is back: Issue in delineating the radiation fieldin Hodgkin's disease. Ann Oncol 13:79â83, 2002.