Hodgkin's Lymphoma in Younger Patients: Lessons Learned on the Road to Success
Despite significant improvements in the treatment of Hodgkin's lymphoma over the past 2 decades, physicians continue to face dilemmas in therapy for the disease, and many cured patients live with complications of treatment. Newer therapeutic options are still needed for the disease, to minimize complications and to improve the treatment of patients in relapse. This review considers the treatment of Hodgkin's lymphoma in younger patients, addressing such issues as which patients with early-stage disease may require radiotherapy, what prognostic factors provide information that can affect treatment choices in patients with advanced disease, and what we have learned about treatment complications in this setting.
Despite significant improvements in the treatment of Hodgkin's lymphoma over the past 2 decades, physicians continue to face dilemmas in therapy for the disease, and many cured patients live with complications of treatment. Newer therapeutic options are still needed for the disease, to minimize complications and to improve the treatment of patients in relapse. This review considers the treatment of Hodgkin's lymphoma in younger patients, addressing such issues as which patients with early-stage disease may require radiotherapy, what prognostic factors provide information that can affect treatment choices in patients with advanced disease, and what we have learned about treatment complications in this setting.
Hodgkin's lymphoma (HL) is a rare, curable malignancy, and investigators have made remarkable improvements in treatment of the disease over the past 20 years. Still, physicians face dilemmas in therapy for the disease, and many cured patients live with complications of treatment. The type of therapy given to these patients determines long-term survival rates. The young patient with HL treated in the past had a greater chance of dying of some malignancy other than HL, and at an earlier age than someone who has not had the disease. Relapse therapy can cure approximately 50% of patients, depending on patient- and disease-related features, and failure of initial therapy, once considered an ominous sign, may not be such a poor feature any longer.
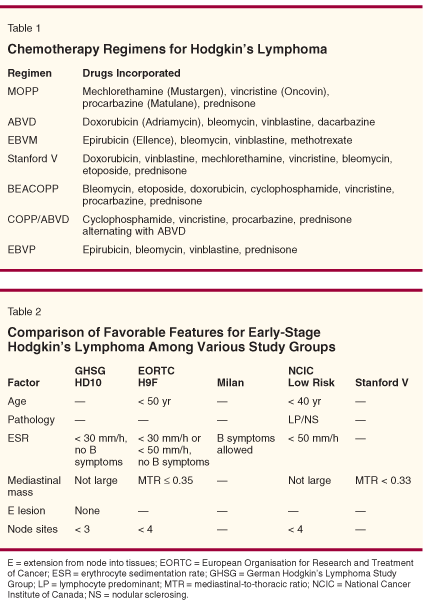
Newer therapy options are needed for the disease-not only novel initial therapy designed to treat the patient with minimal complications, but also therapy for those in relapse. In this review, we consider the treatment of HL in younger patients, first evaluating recent clinical trials for early-stage disease, with a special focus on which patients may still need radiotherapy (RT). For advanced disease, prognostic factors may provide information for those wishing to use treatment other than the ABVD regimen (Table 1) or other standard chemotherapy regimens. Finally, investigators should develop methods that minimize the complications of therapy.
Treatment of Early-Stage HL
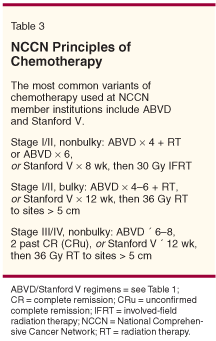
Most US studies of HL in the past were performed in patients treated with radiotherapy followed by laparotomy. Investigators used these results to form the current staging system for HL. However, following the development of MOPP chemotherapy (Table 1), it became possible to cure patients with chemotherapy, and laparotomy became unnecessary. At the same time, chemotherapy and adjuvant RT was the standard of care in Europe. For these reasons, prognostic-factor systems developed by investigators in Europe and the United States have differed, making comparisons between trials difficult (Table 2).
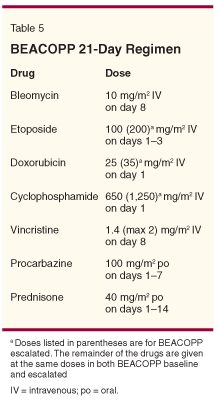
Regardless, MOPP and therapies that have relied on MOPP-based regimens have gradually given way to treatment with ABVD and similar regimens, not only in the United States, but also in Europe (Table 1). Recent trials suggest that ABVD or variants of this combination with or without RT may still be the best therapy for early-stage HL, regardless of the type of disease presentation, and the potential for complications of therapy may ultimately direct choices of treatment. Guidelines for therapy also still include ABVD as a standard (Table 3).
Favorable Presentations
• ABVD and RT-Lymphocyte-predominant HL is often a disease of younger patients and differs from classic variants of the disease in important ways. In the World Health Organization classification, it is a separate entity (Table 4). The classic malignant cell of lymphocyte-predominant HL is a "popcorn" cell, which is CD20-positive and CD15- and CD30-negative, as opposed to findings for classic variants. Patients rarely present with advanced disease, and RT alone is an excellent treatment choice.[1] Also, because of CD20 expression on the cells, rituximab (Rituxan) may be good therapy for the disease. However, there will always be questions as to the best treatment for this disease because of its rarity and the good results obtained with most treatment choices.

Most patients with early stages of classic HL, even those with favorable presentations, receive chemotherapy in the modern era, although investigators still disagree on what chemotherapy and how many cycles should be administered, and on the use of RT. In one study, which treated patients with stage I/II HL with four cycles of ABVD and either extended-field or involved-field RT, freedom-from-progression rates for both arms were 95% at 9 years, and unaffected by various prognostic factors.[2] However, grade 3/4 neutropenia occurred in 10%, infections in 11% (including hepatitis in two, one of whom died), and myocardial infarction in one patient. Late complications included a low ventricular ejection fraction in one, more than 20% decline in diffusing lung capacity (DLCO) in 25% of the patients after RT (which persisted in six), and acute myelogenous leukemia (AML) and colon and breast cancers in one patient each.
Others have reported that ABVD provides better event-free survival results than EBVM (Table 1) when either is followed by extended-field RT in patients with stage I-III HL.[3] Because of relapse therapy, however, overall survival rates were both 90%. Still others have tested the value of epirubicin (Ellence)-based therapy for HL, in an attempt to eliminate RT from the treatment program.[4] These investigators treated patients with six cycles of EBVP (Table 1). Those who entered complete remission then received either 36 Gy involved-field RT, 20 Gy involved-field RT, or no RT. EBVP induced complete remission in 79% of these patients, and 3-year failure-free survival results were 86% for those receiving 36 Gy, 88% for those receiving 20 Gy, and only 68% for those receiving no RT. Overall survival results were excellent on all three arms. However, in the subsequent H10 study, the investigators decided to treat patients with ABVD and 30 Gy involved-field RT or no RT, with the decision to give RT based on positron-emission tomography (PET) findings.
The German Hodgkin's Lymphoma Study Group (GHSG) has also treated young patients with early-stage, favorable HL in a series of trials.[5] In an early study, failure-free survival results with two cycles of ABVD followed by extended-field RT were superior to those reported for extended-field RT alone. Therefore, in the HD10 study, patients with favorable HL received four or two cycles of ABVD followed by 30 or 20 Gy of involved-field RT. In this study of 1,131 patients, those who received four cycles of ABVD experienced more toxicity than did those who had two; however, failure-free and overall survival results were similar on all four arms, possibly establishing a new standard of care for these patients. Following a critical central review, however, the GHSG investigators found that 49% of the patients in this study needed changes in RT fields and doses, suggesting that strict adherence to RT practice may be difficult in such studies. Most importantly, administration of two ABVD cycles has not been used to treat patients with unfavorable HL.
In the HD13 trial, patients with favorable HL received two cycles of ABVD, two of ABV (ABVD without dacarbazine), two of AVD (ABVD without bleomycin), or two of AV (ABVD without bleomycin or dacarbazine), followed by 30 Gy of involved-field RT. So far, complete remission rates are 98% or greater in all four arms. Three patients have died of bleomycin pulmonary toxicity, and one has developed AML. In the forthcoming HD16 trial, patients will receive two or three cycles of AV, depending on PET results, followed by BEACOPP therapy (Tables 1 and 5) if the PET is still positive after AV. No RT will be administered in this trial.
• Chemotherapy Alone-Rueda et al treated patients with stage I/II HL using six cycles of ABVD, reserving RT only for those with unfavorable presentations.[6] The 5-year progression-free survival and overall survival results for those with favorable features were 90% and 98%.[7] Straus et al also treated patients with nonbulky, stages I/IIA HL with six cycles of ABVD with or without RT. They found no differences in TTP or overall survival results on these two arms, suggesting that ABVD alone is adequate therapy for those with this type of HL.[8] More importantly, these investigators had to discontinue bleomycin in 22% of the patients due to lung toxicity, and one patient died. This recognized problem with bleomycin toxicity may ultimately change the standard of care for HL in the United States and Europe.
Other investigators still think that RT is necessary for favorable stage I/II HL. In one study, "low-risk" patients with stage I-IIIA disease received four to six cycles of ABVD or subtotal lymphoid irradiation, while those with "high-risk" disease received four to six cycles of ABVD or two cycles of ABVD followed by subtotal lymphoid irradiation.[9] Patients who received ABVD alone or RT as part of their therapy had 5-year progression-free survival rates of 87% and 93% (P = .006). However, event-free and overall survival results were similar.
Despite these findings, many researchers consider ABVD alone a good choice for "low-risk" patients, avoiding risks of second malignancies and other complications of RT. Still, investigators must ask whether we need confirmation of the results reported for ABVD alone, whether treatment should be based on PET findings, whether we can effectively eliminate bleomycin from the ABVD program, and how RT can be made safer.
Unfavorable Presentations
The definition of adverse prognostic factors for early-stage HL varies among different centers. Multiple investigators have reported that the bulky mediastinal mass is an adverse factor for patients with HL.[10-12] However, in different studies, investigators have reported that other features of the disease or the patient may be important independent prognostic factors for patients with early-stage HL, including age, male gender, mixed-cellularity/lymphocyte-depleted histology, extent of nodal involvement, a high mediastinal-to-thoracic ratio, the presence of B symptoms, an elevated erythrocyte sedimentation rate, and extranodal involvement. These differences demonstrate the problems encountered when deciding upon optimal therapy for these patients. Fortunately, investigators have recently suggested that even patients with bulky mediastinal disease may receive limited therapy.
How Much Chemotherapy Is Necessary?
In a series of studies of treatment for advanced HL conducted by German investigators, failure-free and overall survival results following BEACOPP were better than those following COPP/ABVD (Table 1). However, for early-stage disease, results for ABVD and BEACOPP may be similar.
In a European study, patients with unfavorable HL received four or six cycles of ABVD or four of BEACOPP (baseline), followed by involved-field RT.[4] Complete remission rates for these three treatments were 71%,74%, and 60%, and 3-year progression-free survival results were 92%, 94%, and 80%, respectively. Toxicity was much more common with BEACOPP, resulting in hospitalization of 22% of patients receiving this regimen. This study confirmed that four cycles of ABVD followed by RT is a good treatment for poor-risk patients, even though there is still a risk of pulmonary toxicity with this regimen.
In another European trial, a four-armed study randomized four cycles of ABVD and four of BEACOPP followed by 20 or 30 Gy of RT.[13] As in earlier trials, toxicity was more common in those receiving BEACOPP or 30 Gy RT, and complete remission, failure-free survival, and overall survival results were unaffected by treatment choice. This study not only confirmed that four cycles of ABVD followed by RT is an excellent therapy for high-risk HL, but also that lower doses of RT are just as effective as higher doses.
Reducing Risk of Pulmonary Toxicity by Eliminating Bleomycin From ABVD
Multiple investigators have reported toxicity related to bleomycin. Straus and colleagues deleted bleomycin from ABVD therapy in 22% of their patients, and one died because of this drug.[8] Bonadonna et al reported that 14% of their patients developed interstitial fibrosis as a complication of cure using ABVD and RT.[2] In a study by Hirsch et al, bleomycin significantly affected pulmonary function studies, especially DLCO and forced vital capacity (FVC) values, which often persisted after therapy. These investigators also reported on the reduction or omission of bleomycin in 25% of their patients, and one died from ABVD.[14]
Recent trials have challenged the need for bleomycin in ABVD. In the German trials of therapy for early-stage HL, results with AV and AVD were similar to those reported with ABVD.[5] In a retrospective analysis of Cancer and Leukemia Group B (CALGB) trials, Canellos et al reported that the risk of recurrent HL was the same whether one had received full-dose bleomycin or not.[15] Based on these and other observations, two groups are currently studying novel regimens that do not include bleomycin: AVG (doxorubicin [Adriamycin], vinblastine, gemcitabine [Gemzar]) without RT for patients with favorable stage I/II HL, and PVAG (prednisone, vinblastine, doxorubicin, gemcitabine) for unfavorable stage IIB HL. These investigators hope to confirm that bleomycin is unnecessary in the treatment of HL.
Prognostic Models for Treating Advanced HL: How Much Older Is Old?
In the influential study reported by Canellos et al, patients with advanced HL were randomized to therapy with MOPP or ABVD. Failure-free survival results were better for those who received ABVD, although overall survival results were similar.[16] Low-risk patients were those under age 50 with no or only one site of extranodal disease, whereas those with a high risk of failure included those over 50 who received MOPP therapy. For this reason, both MOPP and ABVD may be considered effective therapy for younger patients; however, MOPP was considered more toxic and abandoned in the United States for all of these patients.
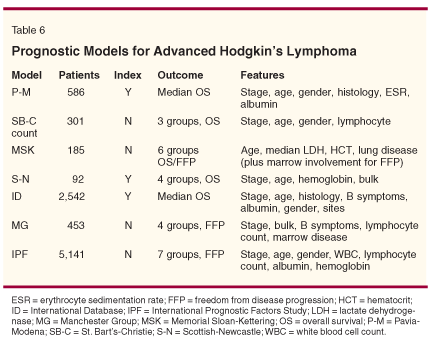
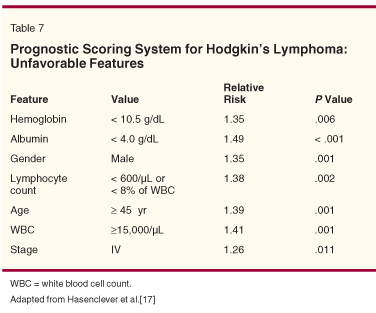
Investigators have reported various models that can predict the chance of progressive disease and death for patients with advanced HL following MOPP therapy (Table 6). On the other hand, Hasenclever et al reported the results of a retrospective study that could predict prognosis in patients receiving ABVD (Table 7).[17] They used seven factors to create a model that predicts the risk of relapse or death in these patients, most of whom were under 60. An age of 45 years or greater was an important feature of this plan. Assigning one point for each feature described, 5-year progression rates were 20% for those with a score of 0 and 60% for those with a score of 5 or greater. Other prognostic factors and tumor-related features, including microarray studies, may be important in detecting which patients have a high risk of relapse or death, but clinicians will continue to rely on clinical features to predict outcomes for their patients until such factors and special studies become more commonly accepted and available.[18-21]
Is There Anything Better Than ABVD for Advanced HL?
Stanford V
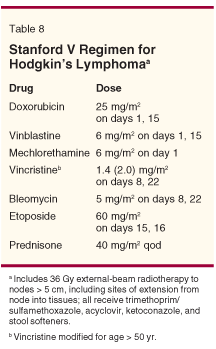
As previously described, ABVD was better than MOPP for two reasons in the study reported by Canellos et al: the regimen produced less toxicity and better failure-free survival results. Importantly, no patient received RT as initial treatment for the disease. However, Horning et al developed Stanford V, a novel regimen in which patients with advanced HL received weekly chemotherapy followed by involved-field RT to all sites of originally detected nodal disease (Table 8).[22] In an update of their study, low-risk patients received eight weekly chemotherapy doses followed by 30-Gy involved-field RT, whereas high-risk patients received 12 weeks of therapy followed by 36-Gy involved-field RT to bulky sites and 30-Gy involved-field RT to all other sites. Patients also received antibiotics for the prevention of fungal and viral infections and Pneumocystis carinii pneumonia. Eight-year progression-free survival results for these patients ranged from 86% to 98%, and overall survival results were all over 90%; no patient has developed leukemia to date.
Because of this successful trial, an Intergroup study in the United States is now treating patients with six courses of ABVD (with RT given only to those with bulky mediastinal disease) or Stanford V followed by RT as in the original trials. Any patient with HL is eligible for this trial, regardless of the International Prognostic Score (IPS). The investigators plan to evaluate not only failure-free survival, overall survival, and freedom from progression results, but are also tracking pulmonary function, second malignancies, and reproductive capability.
Others have studied the Stanford V regimen, and have found it particularly effective for certain patients. In the study reported by Yahalom et al, results varied according to the IPS.[23] At 3 years, those with an IPS of 0 to 3 had failure-free and overall survival rates of 86% and 95%, compared with only 50% and 75% for those with an IPS of 4 to 7. Important features determining time to treatment failure included stage IV disease and pretreatment serum albumin.
Chisesi et al have also treated advanced HL with Stanford V or ABVD, but in their study, RT was considered optional.[24] ABVD was significantly less toxic than Stanford V, yet complete remission rates for ABVD and Stanford V were 89% and 76% (P < .01). Five-year failure-free survival rates were 78% and 51% (P < .01). Potential reasons for the lack of disease control with Stanford V may have been the optional nature of the planned RT. Based on these trials, National Comprehensive Cancer Network guidelines include both ABVD and Stanford V as appropriate therapy for patients with HL. However, only the ongoing randomized trial will prove which regimen is less toxic and which is more effective.
BEACOPP: Intensity for Improvement at a Price
As previously mentioned, BEACOPP is a novel regimen developed by the GHSG to provide an intensive alternative to COPP/ABVD, a combination that substitutes cyclophosphamide for mechlorethamine in the MOPP regimen, and alternates it with ABVD. BEACOPP delivers all drugs within 14 days.[25,26] The GHSG studied three treatments: COPP/ABVD, BEACOPP-baseline (BEAb) and BEACOPP-escalated (BEAe). The escalated regimen has higher doses of etoposide, doxorubicin, and cyclophosphamide than are in the BEAb program (Table 5). All patients received eight chemotherapy cycles followed by involved-field RT.
Early on in this study, it became clear that COPP/ABVD was a significantly inferior treatment, and this arm was closed to enrollment. With follow-up, the complete remission and 3-year freedom from treatment failure rates were best for those who received BEAe (96% and 93%), and those with BEAb were better than those for COPP/ABVD. When analyzed by IPS criteria, BEAe was the best regimen for all groups. Acute hematologic toxicity was more common with BEAe, although infection occurred in only 3%. Despite the improved results reported with BEAe and BEAb, these regimens have not gained great acceptance in the United States. One of the possible drawbacks is the potential for late toxicity. Thirteen patients have developed AML or myelodysplastic syndromes (MDS) after BEA, compared to only one treated with COPP/ABVD. BEAb is also too toxic for older patients and should only be tried in patients under 65.[27]
More recently, Diehl et al have studied the need for RT following BEACOPP for patients with advanced HL.[28] In this study, patients received eight cycles of BEAe or four of BEAe followed by four of BEAb, with or without involved-field RT. The complete remission, freedom from treatment failure, and overall survival rates were similar on all four arms. In the most recent trial, patients are receiving BEAe or BEAb, but the use of RT is based on PET results: if the patient tests positive for residual disease following completion of therapy, investigators administer RT, but if PET is negative, RT is omitted. This study questions the role of RT in the treatment of advanced HL, but only in the setting of positive PET following BEACOPP. Whether results can be extrapolated to other regimens remains to be determined.
Complications of Therapy for HL: Lessons Learned
Secondary Malignancies: Risks and Potential Causes
Salloum et al described the risk of complications of chemotherapy and RT for early-stage HL. At 4 years from the start of therapy, almost 20% of patients in this series had developed significant problems, whereas less than 5% had died of HL.[29] The main non-HL causes of death included malignancies, cardiopulmonary disease, and infectious complications.[30] Investigators have long been aware that secondary cancers can occur following therapy for HL. MDS and AML are induced by alkylating agents, and secondary solid tumors are associated with RT. However, secondary leukemias have actually been associated with most drugs used to treat HL.
Andrieu et al found that AML occurred in 2 of 192 patients receiving ABVD.[31] Van Leeuwen et al also found higher risks for almost all cancers compared to those reported for the general population, including not only lung and breast carcinomas (tending to occur within radiotherapy fields), but also a wide variety of other tumor types (occurring outside of RT fields).[32] Schoenfeld et al found that the unadjusted risk of AML was 20 times that of the risk in the general population, at a median time of 5.9 years from the diagnosis of HL.[33] Men had a higher risk than did women, and those at least 35 years old at HL diagnosis had the highest risk-20.5 times that for a matched population. These investigators also found that the risk for AML was higher for those treated from 1970 through 1984, compared to those treated from 1985 through 2001, regardless of what type of therapy was administered. Thus, there may be an inherent risk of AML in patients with HL, aside from the treatment effect, suggesting that only long-term follow-up of various studies can detect the occurrence of this complication.
Cardiovascular and Infectious Problems: Incompletely Studied Complications
Most investigators assume that the risk of death from cardiac disease is higher in patients who receive mediastinal RT. In a study of ABVD and EBVM followed by RT, Le Maignan et al found that 19% of those treated with mediastinal RT suffered cardiac events, compared to none who did not receive RT (P = .003).[3] More recently, Dores et al-studying the same large group of patients evaluated by Schoenfeld-found that solid tumors caused more deaths in patients who received RT or combined-modality therapy, compared to chemotherapy alone.[34] The relative risk for cardiovascular deaths, including cerebrovascular accidents and cardiac causes, was 7 in those receiving RT, 5.1 for those treated with combined-modality therapy, and 3.7 for those receiving chemotherapy only. However, the risk of death due to infection was greatest in those who received chemotherapy alone.
These studies demonstrate that long-term evaluation and counseling of patients with HL remains an important part of therapy for these patients. Such measures may guide us in our evaluation of the potential for serious complications following any therapy for HL.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Ha C, Kavadi V, Dimopoulos M, et al: Hodgkin's disease with lymphocyte predominance: Long-term results based on current histopathologic criteria. Int J Radiat Oncol Biol Phys 43:329-334, 1999.
2. Bonadonna G, Bonfante V, Viviani S, et al: ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin's disease: Long-term results. J Clin Oncol 22:2835-2841, 2004.
3. Le Maignan C, Desablens B, Delwail V, et al: Three cycles of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) or epirubicin, bleomycin, vinblastine, and methotrexate (EBVM) plus extended radiation therapy in early and intermediate Hodgkin disease: 10-year results of a randomized trial. Blood 103:58-66, 2004.
4. Noordijk E, Thomas J, Ferme C, et al: First results of the EORTC-GELA H9 randomized trials: The H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin's lymphoma (HL) (abstract 6505). J Clin Oncol 23(16S):561s, 2005.
5. Diehl V, Brillant C, Engert A, et al: HD10: Investigating reduction of combined modality treatment intensity in early stage Hodgkin's lymphoma: Interim analysis of a randomized trial of the German Hodgkin Study Group (GHSG) (abstract 6506). J Clin Oncol 23(16S):561s, 2005.
6. Rueda A, Marquez A, Guma J, et al: Treatment of stage I and II Hodgkin's lymphoma with ABVD chemotherapy: Results after 7 years of a prospective study. Ann Oncol 15:1798-1804, 2004.
7. Dominguez R, Marquez A, Guma J, et al: Treatment of stage I and II Hodgkin's lymphoma with ABVD chemotherapy: Results after 7 years of a prospective study. Ann Oncol 15:1798-1804, 2004.
8. Straus D, Portlock C, Qin J, et al: Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood 104:3483-3489, 2004.
9. Meyer R, Gospodarowicz M, Connors J, et al, for the National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group: Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin's lymphoma. J Clin Oncol 23:4634-4642, 2005.
10. Colonna P, Jais J, Desablens B, et al: Mediastinal tumor size and response to chemotherapy are the only prognostic factors in supradiaphragmatic Hodgkin's disease treated by ABVD plus radiotherapy: Ten-year results of the Paris-Ouest-France 81/12 trial, including 262 patients. J Clin Oncol 14:1928-1935, 1996.
11. Longo D, Glatstein E, Duffey P, et al: Alternating MOPP and ABVD chemotherapy plus mantle-field radiation therapy in patients with massive mediastinal Hodgkin's disease. J Clin Oncol 15:3338-3346, 1997.
12. Soriano J, Dawson L, Kaminski M, et al: Low dose versus high dose radiation therapy in patients with bulky mediastinal Hodgkin's disease (abstract 1171). J Clin Oncol 20:293a, 2001.
13. Klimm B, Engert A, Brillant C, et al: Comparison of BEACOPP and ABVD chemotherapy in intermediate stage Hodgkin's lymphoma: Results of the fourth interim analysis of the HD 11 trial of GHSG (abstract 6507). J Clin Oncol 23(16S):561s, 2005.
14. Hirsch A, Vander Els N, Straus D, et al: Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function in early-stage Hodgkin's disease. J Clin Oncol 14:1297-1305, 1996.
15. Canelllos G, Duggan D Johnson J, Niedzwiecki D: How important is bleomycin in the adriamycin + bleomycin + vinblastine + dacarbazine regimen? J Clin Oncol 22:1532-1533, 2004.
16. Canellos G, Anderson J, Propert K, et al: Chemotherapy of advanced Hodgkin's disaese with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 327:1478-1484, 1992.
17. Hasenclever D, Diehl V: A prognostic score for advanced Hodgkin's disease: International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med 339:1506-1514, 1998.
18. Dimopoulos M, Cabanillas F, Lee J, et al: Prognostic role of serum beta 2-microglobulin in Hodgkin's disease. J Clin Oncol 11:1108-1111, 1993.
19. Visco C, Vassilakopoulos T, Kliche K, et al: Elevated serum levels of IL-10 are associated with inferior progressioin-free survival in patients with Hodgkin's disease treated with radiotherapy. Leuk Lymphoma 45:2085-2092, 2004.
20. Gobbi P, Bruglia C, Di Giulio G, et al: The directly measurable tumor burden (TB) is the main prognosticator in Hodgkin's disease (abstract 203). Ann Oncol 13(suppl 2):62, 2002.
21. Montalban C, Garcia J, Abraira V, et al: Influence of biologic markers on the outcome of Hodgkin's lymphoma: A study by the Spanish Hodgkin's Lymphoma Study Group. J Clin Oncol 22:1664-1673, 2004.
22. Horning S, Hoppe R, Breslin S, et al: Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: Mature results of a prospective randomized trial. J Clin Oncol 20:630-637, 2002.
23. Yahalom J, Edwards-Bennett S, Jacobs J, et al: Stanford V and radiotherapy for advanced and locally extensive Hodgkin's disease (HD): The Memorial-Sloan-Kettering Cancer Center (MSKCC) experience (abstract 1459). Blood 102:402a, 2003.
24. Chisesi T, Federico M, Levis A, et al: ABVD versus Stanford V versus MEC in unfavorable Hodgkin's lymphoma: Results of a randomised trial. Ann Oncol 13(suppl):S102-S106, 2002.
25. Diehl V, Franklin J, Pfreundschuh M, et al: Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med 348:2386-2395, 2003.
26. Diehl V, Franklin J, Sieber M, et al: Dose-escalated BEACOPP chemotherapy improves failure-free survival in advanced Hodgkin's disease: Updated results of the German Hodgkin's Lymphoma Study Group (abstract 2474). Blood 95(suppl 1):96, 2005.
27. Ballova V, Ruffer J, Haverkamp H, et al: A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin's disease comparing BEACOPP baseline and COPP-ABVD (study HD9 elderly). Ann Oncol 16:124-131, 2005.
28. Diehl V, Schiller P, Engert A, et al: Results of the third interim analysis of the HD12 trial of the GHSG: 8 courses of escalated BEACOPP versus 4 escalated and 4 baseline courses of BEACOPP with or without additive radiotherapy for advanced stage Hodgkins lymphoma (abstract 85). Blood 102:27a, 2003.
29. Salloum E, Doria R, Farber L, et al: Combined modality therapy in previously untreated patients with advanced Hodgkin's disease: A 24-year follow-up study. Ca J Sci Am 1:267-278, 1995.
30. Aleman B, van den Belt-Dusebout A, Klokman W, et al: Long-term cause-specific survival of patients treated for Hodgkin's disease (abstract 216). Ann Oncol 13(suppl 2):65, 2002.
31. Andrieu J, Desablens B, Delwail V, et al: High rate of second primary neoplasms (SPN) among Hodgkin's disease (HD) patients (Pts) treated with ABVD, a dacarbazine (DTIC)-containing chemotherapy (CT): Eight year results of a randomized trial of the GOELAMS Group (abstract 1128). Proc Am Soc Clin Oncol 20:283a, 2001.
32. Van Leeuwen F, Klokman W, Hagenbeek A, et al: Second cancer risk following Hodgkin's disease: A 20-year follow-up study. J Clin Oncol 12:312-325, 1994.
33. Schoenfeld S, Gilbert E, Dores G, et al: Acute leukemia (AL) following Hodgkin's lymphoma (HL): A population-based study of over 38,000 patients (abstract 6510). J Clin Oncol 23(16S):562s, 2005.
34. Dores G, Schoenfeld S, Chen D, et al: Long-term cause-specific mortality among 41,146 one-year survivors of Hodgkin lymphoma (HL) (abstract 6511). J Clin Oncol 23(16S):562s, 2005.