Sexual health is an important aspect of human life, and cancer does not (and should not) change that. Data suggest that issues related to sexual function are quite common among women treated for cancer. However, clinicians often spend little to no time on the topic. This article provides a concise summary on the importance of sexual health among women treated for cancer, as well as an approach that general cancer clinicians can adopt in order to normalize sexual health issues for their patients. Finally, we provide an overview of sexual health therapeutics available in the United States and in Europe.
Introduction
In 2015, 1,658,370 new cases of cancer were diagnosed and 589,430 persons died of cancer in the United States.[1] However, the proportion of people living with and surviving cancer is growing. More than 7.5 million out of 14.5 million cancer survivors in the United States are women, and that number is expected to grow to 9,602,590 by 2024. The increase in survival rates reflects both earlier diagnosis and improvement in treatment.[1] Despite the data showing that most survivors have a good prognosis, current treatments can result in problems, including symptoms related to sexual health.
In 2007, Beckjord and Campas documented significant disruption in sexual quality of life that was the result of treatments and of emotional distress-rather than of age-in women with a diagnosis of breast cancer.[2] Estimates of the incidence of sexual dysfunction range from 30% to 100% among female cancer survivors, depending on the population queried and on how sexual dysfunction is characterized.[3,4] Sexual dysfunction affects both women with illness and women in the general population, and discussing sexual health in both populations remains difficult-for both patients and providers.
What Are the Components of Sexual Function in Women?
Female sexual function is not exclusively a physiologic phenomenon; it is influenced by both physiologic and psychosocial factors. This has been best illustrated in a model proposed by Rosemary Basson, in which sexual health is characterized as a self-propagating circle of wanting intimacy, stimuli, arousal, desire, and satisfaction; importantly, physical acts, such as intercourse, are not inherent in the concept of sexual function.[5] However, recognizing that some women may not be motivated by a desire for intimacy, Basson has also proposed an alternative model in which the intimacy component can be “bypassed,” and in which sexual function is motivated by hunger.[6]
What Constitutes Female Sexual Dysfunction?
Sexual dysfunctions are characterized by a disturbance in sexual desire and by disturbances in the psychological and physiologic changes involved in the sexual response.
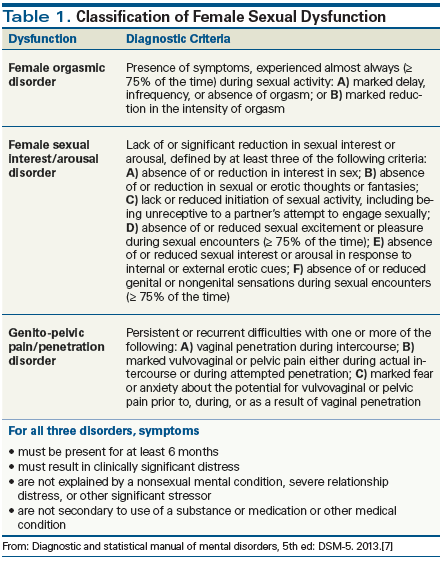
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) recognizes three types of sexual dysfunction in women: sexual interest/arousal disorder, genito-pelvic pain/penetration disorder, and female orgasmic disorder.[7] However, the presence of symptoms is not sufficient for the diagnosis (Table 1). Rather, symptoms must be present for a minimum of 6 months and must be severe enough to cause distress or the inability to respond sexually or experience sexual pleasure. Finally, each type of sexual dysfunction should be subcategorized based on two factors: whether it is “lifelong vs acquired,” and whether it is “generalized vs situational.” It should be noted that a diagnosis of female sexual dysfunction must be arrived at after careful consideration of a differential diagnosis that includes other possible etiologies (eg, drug or substance side effects, or consequences of another disease process).[7]
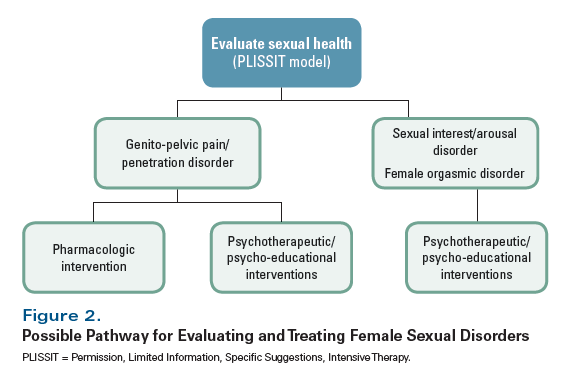
Evaluating Female Cancer Survivors for Sexual Health Issues
Despite the impact of cancer treatments on female sexual health, this issue is often ignored, probably for a variety of reasons. For example, patients may be uncomfortable discussing such a sensitive topic with their oncologist. In addition, oncologists may not have the background, knowledge, or comfort level to engage in discussions of sexual health.[8] In a recent review, Halley et al suggested that there were three main barriers that needed to be overcome in order for women with breast cancer to be able to discuss and get help with their issues in sexual functioning: 1) the assumption on providers’ part that sexuality is primarily a physical issue, rather than a global one; 2) the fact that patients-in particular, those being treated at cancer-specific centers-have difficulty in identifying the right person to ask for an evaluation of sexual problems; and 3) patients may migrate away from cancer care providers after the end of treatment or due to other factors, and as such, may not be able to discuss it with them after sexual issues arise.[9]
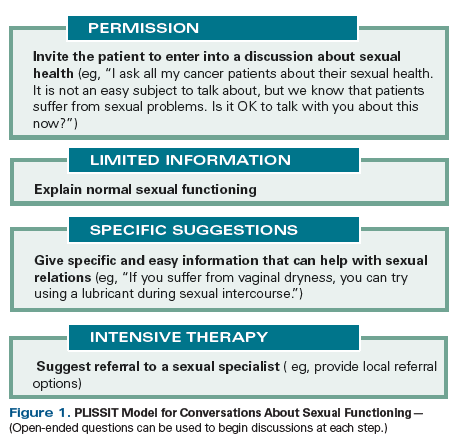
One suggested approach to initiating a discussion of sexual health is the PLISSIT (“Permission, Limited Information, Specific Suggestions, and Intensive Therapy”) model (Figure 1).[10,11] However, it is also important to ensure that a standard medical history is obtained, with particular attention paid to other medical problems; medications; and social conditions, including issues related to a patient’s partner (where applicable) and to stressors that can potentially affect sexual health.[12]
Sexual health can also be evaluated using questionnaires designed for patient-reported outcomes. An example is the Brief Sexual Symptom Checklist for Women (SSFF-A), which evaluates three domains in women (libido, sexual activity, and sexual satisfaction). One of the most widely used such questionnaires is the Female Sexual Function Index (FSFI), which consists of 19 items that cover 6 domains in female sexuality: desire, arousal, lubrication, orgasm, satisfaction, and pain.[13] It should be noted that the relevance of the FSFI is limited in women who are not actively engaged in sexual activity, especially since the recall period is the preceding 4 weeks.[14] In addition, some research indicates that the FSFI does not take into consideration certain psychological issues that are relevant in women with cancer, such as the role of the partner and a woman’s precancer sexual functioning.[15] In 2015, Bartula and Sherman reported on an adaptation of the FSFI for use in women with breast cancer (FSFI-BC); this version added cancer and distress subscales and included items applicable to women who are not sexually active. The investigators reported that the FSFI-BC had favorable psychometric properties and was acceptable for use in this population, irrespective of whether or not women were currently sexually active.[16] Whether the FSFI-BC can be utilized in women with other types of cancers is an open question.
Treatment Strategies for Female Sexual Dysfunction
Various types of interventions are available for the treatment of sexual dysfunction in women. The multifactorial nature of female sexual concerns requires a multidisciplinary therapeutic approach.[17] What follows is a brief summary of both nonpharmacologic and pharmacologic therapies, with a focus on those currently available in the United States and/or Europe.
Psychotherapeutic and psycho-educational interventions
There are a number of psychotherapy approaches for women with sexual dysfunction, including cognitive behavioral therapy, sensate focusing, counseling, and relationship (or marital) therapy.[18] These approaches aim to address fear or anxiety around sexual activity, to improve intimacy, and to reduce the overall severity of sexual dysfunction. In addition, for women with partners, an aim may be to address communication skills between partners.[19,20] In 2017, Rottmann et al analyzed the correlation between the individual’s sexual functioning and that of the couple, and reported that satisfaction with sex life in couples needs to be seen as a couple issue. This stresses the importance of taking the partner into account when addressing issues of sexual functioning.[21]
Pelvic floor muscle training
Pelvic floor muscles give structural support to the pelvic organs (urethra, vagina, and rectum). Surgery and radiotherapy can cause sexual pain through various effects, ranging from anatomic changes to more subtle adverse effects on skin, mucosa, muscle, connective tissue, nerves, and lymphatic vessels.[22] Moreover, pelvic floor dysfunction may be an indirect outcome of various cancer treatments that reduce vaginal elasticity and lubrication (eg, use of antiestrogen preparations). Routine use of rehabilitative postoperative physical therapy increases pelvic blood flow and stretches reactive pelvic floor, facilitating healing of the vagina. Self-massage mobilizes soft tissues and may decrease scar formation, introital narrowing, and superficial dyspareunia.
Certainly, the use of vaginal dilators is important for radiated patients in order to reduce the risk of vaginal stenosis. The goal of dilation is frequent separation of the vaginal walls to minimize formation of adhesions while tissues heal.[23] However, many women affected by cancer encounter physical difficulties, discomfort, and fear when beginning use of vaginal dilators on their own. Success with dilators is higher with the help of expert pelvic floor physical therapists who can give personal instructions.[24]
Therefore, pelvic floor rehabilitation and physical therapy should be considered as a minimally invasive therapy, with the general objectives of remission and/or pain management, by means of restoration of proper parameters of the pelvic floor muscles, and restoration of altered functions (urinary and fecal incontinence, sexual). A multidisciplinary approach seems to be a logical requisite for a correct treatment strategy.
Over-the-counter pharmacologic and device therapies
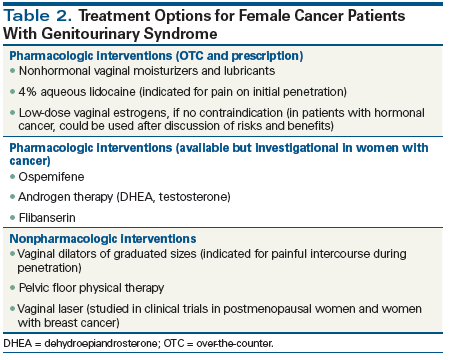
First-line therapies include the use of over-the-counter nonhormonal vaginal moisturizers to improve vulvovaginal atrophy.[25] Several preparations exist, including polycarbophilic preparations and a paraben-free preparation. These should be applied regularly, regardless of intercourse. Regular use of nonhormonal, long-acting vaginal moisturizing agents can decrease vaginal pH to premenopausal levels, although they do not improve the vaginal maturation index. Use of lubricants during vaginal intercourse may also reduce friction-related irritation of atrophic tissue.[25] At least one randomized trial has shown that vaginal moisturizers can be quite effective.[26]
For women who experience pain during penetration, vaginal dilators are often prescribed, although most of the data published on these have been from women treated with pelvic radiotherapy. The authors of a Cochrane review concluded that there were no level 1 data that showed that regular and routine vaginal dilation prevented vaginal stenosis or improved quality of life.[23] However, they acknowledged that observational studies did report those benefits, and thus they argued for carefully crafted trials to better inform the use of vaginal dilators.
Prescription pharmacologic interventions
For many women, sexual dysfunction and menopausal symptoms are intimately related, especially if they have been suddenly thrust into a postmenopausal state as a result of treatment (eg, oophorectomy in women with ovarian cancer, aromatase inhibitor therapy with or without ovarian suppression in women with breast cancer). Therefore, treatment of genitourinary syndrome of menopause (GSM)[25,27] may have the additional advantage of improving sexual function.
Among the pharmacologic options for treatment of GSM, some address vasomotor effects (eg, gabapentin or the selective reuptake inhibitors of serotonin or norepinephrine for hot flashes), while others address vulvovaginal atrophy and dryness.[28] Local estrogen preparations are among the most commonly utilized for GSM, although we acknowledge that for some cancer survivors, especially those with hormone-responsive malignancies, the issue of estrogen therapy remains controversial.[29-31] Nonetheless, while oral estrogen therapy may be contraindicated (eg, in women with hormone receptor–positive breast cancer) vaginal hormonal preparations are acceptable.[32] Indeed, the American College of Obstetricians and Gynecologists has determined that the benefits of local estrogen therapy appear to outweigh the risks of treatment in this population, and thus it is considered a reasonable option to explore with patients, particularly if nonhormonal treatments have been ineffective.[25]
Beyond vaginal estrogen, other hormonal preparations have recently been approved in the United States and/or Europe. Ospemifene is a locally acting selective estrogen receptor modulator that exerts its effects predominantly in the vagina and has demonstrated antiestrogenic activity in preclinical models of breast cancer.[33,34] However, the effectiveness of this agent specifically in women who have been treated for cancer is unknown, as such women were not included in the original randomized trials.[35,36] Because of this, the US Food and Drug Administration (FDA) has cautioned that ospemifene should not be used in women with breast cancer or at high risk for breast cancer. Another agent is vaginal dehydroepiandrosterone (DHEA), which was approved for vulvovaginal atrophy in the United States in 2017.[37] However, as with ospemifene, the randomized trials on which approval of vaginal DHEA was based excluded women with cancer-although some data suggest that this agent is effective in women treated for breast or gynecologic cancer, without causing any significant change in systemic estrogen levels.[38] Testosterone preparations remain investigational for the treatment of sexual dysfunction in women. One randomized trial conducted in the United States of a testosterone preparation vs placebo failed to show improvement in sexual functioning.[39]
KEY POINTS
- Sexual health is an important part of human life and should be addressed in women being treated for cancer.
- Cancer treatment (including surgery, chemotherapy, and hormonal therapy) has short- and long-lasting physical, psychological, and social effects that can interfere with normal sexual function in women.
- Cancer providers should be prepared to address the sexual health of their female patients, or at least should be able to refer them to a locally available expert.
For women who complain of pain at initial penetration, 4% aqueous lidocaine may provide relief. This was demonstrated in a US randomized trial that included 46 breast cancer survivors with estrogen-deficient breast cancer.[40] Compared with the group assigned to placebo, the women who used lidocaine reported significantly less pain during intercourse and significantly lower sexual distress scores. Most notably, of the 20 women in the study who had been abstaining from intercourse, 17 (85%) had resumed comfortable penetrative intercourse after use of lidocaine (open-label lidocaine use was permitted after the randomized portion of the trial).
Finally, for women with low desire and no other signs or symptoms of sexual dysfunction, flibanserin has been approved by the FDA. Flibanserin reportedly acts centrally and increases norepinephrine levels to help stimulate sexual interest.[41] Randomized trials have shown it is effective, although none of these trials have included women with a history of cancer, and in particular, a population taking an antiestrogen preparation.[42,43] While approved for use in the United States, flibanserin is not available in Europe. In addition, it remains to be seen whether it will be effective in cancer survivors.
Vaginal laser
Short-term studies have suggested that laser therapy may result in remodeling of vaginal connective tissue and thickening and improved glycogen storage of the vaginal epithelium in postmenopausal women with vaginal atrophy. Improvement in symptoms of vulvovaginal atrophy (vaginal dryness, burning, itching, and dyspareunia) and in vaginal health index scores have been reported, but long-term studies are needed.[44-46]
A recent study evaluated the efficacy of fractional CO2 laser therapy in breast cancer survivors as a treatment for vulvovaginal atrophy.[47] The study demonstrated a significant improvement in vulvovaginal atrophy and dyspareunia in breast cancer survivors after they underwent laser therapy. The majority of patients (52%) were satisfied after long-term follow-up (mean follow-up, 11 months). No adverse events resulting from fractional CO2 laser treatment occurred. The investigators concluded that treatment with fractional CO2 laser therapy was a reasonable option for breast cancer survivors with contraindications to hormonal treatments.
Conclusions
Sexual dysfunction in women affected by cancer will continue to increase as a result of improvements in cancer treatment and prognosis and the consequent growth in the number of survivors. In addition, associations for cancer survivors are focusing attention on quality of life during and after treatment, including in the area of sexual functioning. Cancer providers must be aware of this emerging issue and should be prepared to address the sexual health of their female patients,[48,49] or at least should be able to refer them to locally available experts.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. American Cancer Society. Cancer facts & figures 2015. Atlanta: American Cancer Society; 2016.
2. Beckjord E, Campas BE. Sexual quality of life in women with newly diagnosed breast cancer. J Psychosoc Oncol. 2007;25:19-36.
3. Desimone M, Spriggs E, Gass JS, et al. Sexual dysfunction in female cancer survivors. Am J Clin Oncol. 2014;37:101-6.
4. Andersen BL. Sexual functioning morbidity among cancer survivors: current status and future research directions. Cancer. 1985;55:1835-42.
5. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-3.
6. Basson R. Women’s sexual dysfunction: revised and expanded definitions. CMAJ. 2005;172:1327-33.
7. American Psychiatric Association, editors. Diagnostic and statistical manual of mental disorders, fifth edition: DSM-5. Washington, DC: American Psychiatric Publishing; 2013.
8. Eden J. Endocrine dilemma: managing menopausal symptoms after breast cancer. Eur J Endocrinol. 2016;174:R71-R77.
9. Halley MC, May SG, Rendle KA, et al. Beyond barriers: fundamental ‘disconnects’ underlying the treatment of breast cancer patients’ sexual health. Cult Health Sex. 2014;16:1169-80.
10. Boswell EN, Dizon DS. Breast cancer and sexual function. Transl Androl Urol. 2015;4:160-8.
11. Annon JS. Behavioral treatment of sexual problems: brief therapy. New York: Harper & Row; 1976.
12. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J. 2009;15:74-7.
13. Maiorino MI, Chiodini P, Bellastella G, et al. Sexual dysfunction in women with cancer: a systematic review with meta-analysis of studies using the Female Sexual Function Index. Endocrine. 2016;54:329-41.
14. Bartula I, Sherman KA. The Female Sexual Functioning Index (FSFI): evaluation of acceptability, reliability and validity in women with breast cancer. Support Care Cancer. 2015;23:2633-41.
15. Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118:4606-18.
16. Bartula I, Sherman KA. Development and validation of the Female Sexual Function Index adaptation for breast cancer patients (FSFI-BC). Breast Cancer Res Treat. 2015;152:477-88.
17. Candy B, Jones L, Vickerstaff V, et al. Interventions for sexual dysfunction following treatments for cancer in women. Cochrane Database Syst Rev. 2016;(2):CD005540.
18. Baucom DH, Porter LS, Kirby JS, et al. A couple-based intervention for female breast cancer. Psychooncology. 2009;18:276-83.
19. Classen CC, Chivers ML, Urowitz S, et al. Psychosexual distress in women with gynaecologic cancer: a feasibility study of an online support group. Psychooncology. 2013;22:930-52.
20. Rowland JH, Meyerowitz BE, Crespi CM, et al. Addressing intimacy and partner communication after breast cancer: a randomized controlled group intervention. Breast Cancer Res Treat. 2009;118:99-111.
21. Rottmann N, Gilså Hansen D, dePont Christensen R, et al. Satisfaction with sex life in sexually active heterosexual couples dealing with breast cancer: a nationwide longitudinal study. Acta Oncol. 2017;56:212-9.
22. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG. 2010;117:522-31.
23. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;(9):CD007291.
24. Brand AH, Do V, Stenlake A. Can an educational intervention improve compliance with vaginal dilator use in patients treated with radiation for a gynecological malignancy? Int J Gynecol Cancer. 2012;22:897-904.
25. The International Menopause Society (IMS). Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2013;16:316-37.
26. Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15:969-7.
27. Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Climacteric. 2014;17:557-63.
28. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11:11-33.
29. Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-8.
30. Kendall A, Dowsett M, Folkerd E, Smith I. Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17:584-7.
31. Ponzone R, Biglia N, Jacomuzzi ME, et al. Vaginal oestrogen therapy after breast cancer: is it safe? Eur J Cancer. 2005;41:2673-81.
32. Biglia N, Bounous VE, Sgro LG, et al. Genitourinary syndrome of menopause in breast cancer survivors: are we facing new and safe hopes? Clin Breast Cancer. 2015;15:413-20.
33. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-6.
34. Pickar JH. The response to ospemifene in normal human breast tissue cultures. Menopause. 2016;23:704-5.
35. Palacios S, Cancelo MJ. Clinical update on the use of ospemifene in the treatment of severe symptomatic vulvar and vaginal atrophy. Int J Womens Health. 2016;8:617-26.
36. Wurz GT, Soe LH, DeGregorio MW. Ospemifene, vulvovaginal atrophy, and breast cancer. Maturitas. 2013;74:220-5.
37. Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23:243-56.
38. Barton DL, Sloan JA, Shuster LT, et al. Impact of vaginal dehydroepiandrosterone (DHEA) on vaginal symptoms in female cancer survivors: trial N10C1 (Alliance). J Clin Oncol. 2014;32(suppl 5S):abstr 9507.
39. Barton DL, Wender DB, Sloan JA, et al. Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido; North Central Cancer Treatment Group protocol N02C3. J Natl Cancer Inst. 2007;99:672-9.
40. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-400.
41. Sang JH, Kim TH, Kim SA. Flibanserin for treating hypoactive sexual desire disorder. J Menopausal Med. 2016;22:9-13.
42. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10:1807-15.
43. Kingsberg SA, Clayton AH, Pfaus JG. The female sexual response: current models, neurobiological underpinnings and agents currently approved or under investigation for the treatment of hypoactive sexual desire disorder. CNS Drugs. 2015;29:915-33.
44. Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-9.
45. Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climateric. 2014;17:363-9.
46. Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO(2) laser in women with vulvovaginal atrophy. Climacteric. 2015;18:219-25.
47. Pieralli A, Fallani MG, Becorpi A, et al. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch Gynecol Obstet. 2016;294:841-6.
48. Dizon DS, Suzin D, McIlvenna S. Sexual health as a survivorship issue for female cancer survivors. Oncologist. 2014;19:202-10.
49. Zhou ES, Nekhlyudov L, Bober SL. The primary health care physician and the cancer patient: tips and strategies for managing sexual health. Transl Androl Urol. 2015;4:218-31.