How the Melanoma Treatment Vemurafenib Causes Growth of Secondary Skin Tumors
In a rare case where the side effect of a cancer treatment can be explained through molecular studies, researchers have identified the reason behind the frequent skin lesion side effect among metastatic melanoma patients that take the newly approved drug vemurafenib.
In a rare case where the side effect of a cancer treatment can be explained through molecular studies, researchers have identified the reason behind the frequent skin lesion side effect among metastatic melanoma patients that take the newly approved drug vemurafenib (Zelboraf).
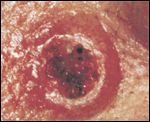
This plate illustrates a keratoacanthoma. Source: The Armed Forces Institute of Pathology
In a large collaborative study published in the New England Journal of Medicine (NEJM), Richard Marais, PhD, Institute of Cancer Research in London, and Dr. Antoni Ribas, Division of Hematology-Oncology at UCLA, have elucidated the mechanism of why 15% to 30% of patients who take vemurafenib to treat their metastatic melanoma develop either cutaneous squamous-cell carcinomas or keratoacanthomas. The lesions are easily excised and patients remain on treatment, but their appearance and longer-term effects are a concern to both clinicians and patients. Marais, Ribas, and colleagues show that it is not likely that the lesions are caused by vemurafenib. Instead, vemurafenib speeds up their development time, as a result of a mutation already present in the skin. This explains why the cutaneous squamous-cell carcinomas and keratoacanthomas affect only about one quarter of patients.
Vemurafenib is the second treatment to be approved for metastatic melanoma in the past year, after a decades-long dry spell of new treatments for the disease. While the first approved treatment, ipilimumab, is an immunotherapy that is broadly applicable to all metastatic melanoma patients, vemurafenib is an oral targeted agent specific for the about 50% of those patients whose melanoma tumors harbor the BRAF V600E mutation. Vemurafenib has greatly improved patient outcomes, resulting in rapid responses in the vast majority of patients and improvement in overall survival compared to standard chemotherapy. Another BRAF inhibitor, dabrafenib is currently in late-stage clinical trials.
In the current NEJM study, the authors analyzed the secondary skin lesion samples of patients that participated in phase I, II, and III vemurafenib clinical trials. The samples were sequenced for genes in the mitogen-activated protein kinase (MAPK) pathway. The BRAF V600E mutation is an activating, driver mutation that activates the MAPK pathway, resulting in constitutive signaling and cancer cell proliferation.
Approximately 60% of the lesions analyzed had a mutation in one of the RAS genes, with HRAS mutations the most frequent. In follow-up cell line studies, BRAF inhibition with vemurafenib resulted in stimulated MAPK signaling in 3 different cell lines with an HRAS mutation, opposite of the effect that occurred when vemurafenib was added to melanoma cells harboring the BRAF V600E mutation.
In contrast to secondary cancers from chemotherapy that take years to develop, the skin lesions among melanoma patients taking vemurafenib crop up on average 10 weeks after the start of treatment, with the earliest ones seen after just 3 weeks. The authors suggest that their circumstantial evidence, including the observation that only a proportion of patients develop the secondary lesions, lead them to the conclusion that treatment with a BRAF inhibitor in patients with a RAS mutation induces the acceleration of squamous-cell cancers rather than the drug causing the RAS mutations.
Addition of a MEK inhibitor on top of the BRAF inhibitor in a mouse skin carcinogenesis model resulted in suppression of the secondary skin lesions in 91% of the mice. However, MEK inhibitor administered after BRAF inhibition treatment did nothing to regress already present lesions. MEK is part of the MAPK pathway, downstream of BRAF. The study authors highlight that combining a BRAF inhibitor with a MEK inhibitor will prevent the side effect. Currently, the combination of a MEK and BRAF inhibitor for metastatic melanoma is in a phase II global clinical trial. Whether patients who take the combination of MEK and BRAF inhibitor have fewer skin and other side effects and less frequent development of resistance is yet to be seen.
In an accompanying editorial, Ashani T. Weeraratna, PhD, of the Wistar Institute in Philadelphia, suggests that patients should be analyzed for RAS mutations prior to starting a BRAF inhibitor regimen for metastatic melanoma.
"This RAS mutation is likely caused by prior skin damage from sun exposure, and what vemurafenib does is accelerate the appearance of these skin squamous-cell cancers, as opposed to being the cause of the mutation that starts these cancers."