Improved Metastatic Uterine Papillary Serous Cancer Outcome With Treatment of Mast Cell Activation Syndrome
A 71-year-old woman not on hormone replacement therapy presented with uterine bleeding. Dilation and curettage revealed complex hyperplasia with atypia, focal clear-cell features, and endocervicitis. Endometrial intraepithelial carcinoma was suspected.
Figure: Photomicrographs of the Patient’s Cancer
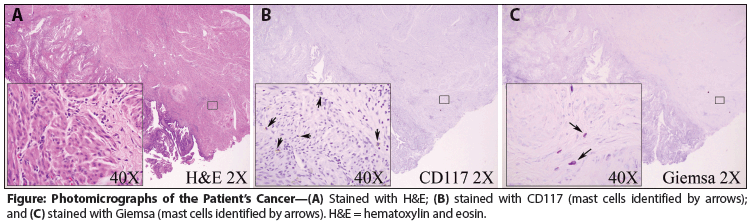
Table: Symptoms and Findings in MCAD
The Case: In May 2010, a 71-year-old woman not on hormone replacement therapy presented with uterine bleeding. Endometrial biopsy was foiled by severe cervical stenosis. Dilation & curettage revealed complex hyperplasia with atypia, focal clear-cell features, and endocervicitis. Endometrial intraepithelial carcinoma was suspected. Frozen section analysis from hysterectomy and bilateral salpingo-oophorectomy revealed noninvasive grade 1 endometrial cancer. Thus, lymphadenectomy was not performed. However, final pathology showed metastatic uterine papillary serous adenocarcinoma (UPSC) with bilateral tubo-ovarian micrometastases. Additional testing showed microsatellite instability but not Lynch syndrome. Positron emission and computed tomography (PET/CT) in August 2010 demonstrated hypermetabolic adenopathy, including a 1.1-cm para-aortic node (fluorodeoxyglucose [FDG] standardized uptake value [SUV] of 3.4), a subcentimeter aortocaval node (SUV, 2.4), and a 1.0-cm node inferior to the aortic bifurcation (SUV, 2.7). Cancer antigen 125 (CA-125) level was 55.9 U/mL.
The patient declined full surgical staging, including pelvic/para-aortic lymphadenectomy. She began carboplatin and paclitaxel (CP), which was well tolerated until superficial venous thrombosis of the right distal great saphenous and right small saphenous veins during cycle 4. Warfarin was started. Restaging PET/CT in December 2010 after cycle 6 demonstrated progression of prior metastatic disease (multiple para-aortic nodes up to 1.2 cm, with SUV of 8.6) and new metastatic disease (external iliac adenopathy up to 0.9 cm, with SUV of 4.7; and paratracheal adenopathy up to 1.3 cm, with SUV of 4.3).
She was started on salvage paclitaxel, doxorubicin, and cisplatin (TAP). In light of her son’s alleged protein S deficiency, she was referred for hematologic consultation; only a modest lupus anticoagulant level was found. Anticoagulation was switched to enoxaparin because of an unstable warfarin response. She was more symptomatic with TAP, reporting abdominal bloating, presyncopal episodes and palpitations (worst in each cycle’s second week, once she was off dexamethasone), soaking sweats, nausea, polyuria, polydipsia, grade 1 peripheral neuropathy, left hip pain on movement, heartburn, constipation, fatigue, and dyspnea, all of which progressed through treatment. Post–cycle 3 PET/CT in March 2011 showed new subpectoral adenopathy (1.7 cm; SUV, 8.2) and a mixed response at other sites (eg, para-aortic adenopathy up to 1.0 cm, SUV of 3.6; paratracheal adenopathy up to 1.5 cm, SUV of 3.3). Diverticulitis in the sigmoid colon was seen and was treated with amoxicillin/clavulanic acid. Abdominal pain improved somewhat. The CA-125 level, which had decreased after the first CP cycle to 24.2 U/mL, was now 15.3 U/mL. TAP was continued. Post–cycle 5 PET/CT in May 2011 again showed a mixed response (eg, subpectoral adenopathy of 1.2 cm × 0.7 cm, SUV of 1.1; paratracheal adenopathy of 1.5 cm, SUV of 3.5; para-aortic adenopathy of 0.6 cm in the short axis, SUV of 1.0). Diverticulitis had resolved. CA-125 reached its lowest level, at 6.9 U/mL, after cycle 7. Post–cycle 8 PET/CT in August 2011 showed minor further response (subpectoral adenopathy of 0.3 cm in the short axis, SUV of 0.6; paratracheal adenopathy of 1.5 cm, SUV of 2.2; para-aortic adenopathy of 0.5 cm in the short axis, SUV of 0.9).
Chemotherapy was stopped. Medroxyprogesterone (MP) at 40 mg twice daily was started. The patient soon noticed worsened fatigue. During evaluation of presyncope in August 2011, she reported many chronic issues. These included hot flashes; chills; migratory pruritus; mild visual anomalies; excessive lacrimation, chronic coryza, and postnasal drip; heartburn; episodic documented hypotension; and life-long unprovoked episodes of hypomania. Mast cell activation disease (MCAD) was considered as a unifying diagnosis. Testing was delayed, but she began a full histamine blockade (famotidine, 40 mg twice daily, and loratadine, 10 mg twice daily); also, enoxaparin was stopped and daily aspirin was increased from 81 mg to 325 mg. Her symptoms all resolved. A month later she stopped aspirin and antihistamines for mast cell (MC) mediator testing. Her symptoms all immediately returned. After testing, she resumed aspirin and antihistamines, and she re-achieved complete remission of her symptoms within a week. Her serum tryptase level was normal, but her serum prostaglandin D2 (PGD2) level was mildly elevated (128 pg/mL [normal, 35–115 pg/mL]), her plasma heparin level was mildly elevated (anti–factor Xa, 0.040 U/mL [normal, 0.000–0.020 U/mL]), and her factor VIII level was moderately elevated (273% [normal, 50% to 150%]).
In view of the clinical history consistent with chronic aberrant MC mediator release, multiple elevated levels of MC mediators (fairly specific PGD2 and heparin levels; also, less specific factor VIII level), absence of any other evident disease better explaining the full range and chronicity of findings, and good response to therapy targeted at MC mediator production and action, the patient met current proposed diagnostic criteria for mast cell activation syndrome (MCAS).[1] Given her normal serum tryptase level and the lifelong chronicity of some of her symptoms that were believed to be attributable to MCAS, systemic mastocytosis (SM) was thought to be unlikely and marrow examination was deferred.[1]
PET/CT in November 2011 showed stable paratracheal adenopathy (1.3 cm in the short axis; SUV, 2.8), subpathologic para-aortic adenopathy with no FDG avidity, and complete resolution of subpectoral adenopathy. PET/CT in May 2012 was similar. In August 2012, the patient developed sterile vaginitis, which responded to an antibiotic, as well as foot pain, which responded to topical nonsteroidal anti-inflammatory drug therapy. A CT scan that month showed no cancer. PET/CT scans in March and September 2013 remained stable. The CA-125 level has been stable (6.9–11.5 U/mL). As of December 2013, the patient has been in sustained remission for 28 months with MP and antihistamines (overall survival has been 44 months since diagnosis). Symptomatic improvement has been fully sustained.
Discussion
This appears to be the first reported case of metastatic UPSC refractory to two lines of chemotherapy, which then responded well after comorbid MCAS was recognized and treated. UPSC is an aggressive postmenopausal nonendometrioid uterine cancer that is often chemoresistant from onset, with low response rates, short response durations, and a 5-year survival rate of 18% for stage IV disease;[2-5] even worse outcomes are expected with disease resistant to two lines of chemotherapy. The relationship between peritumoral MCs and uterine cancer is complex, as reflected in contrasting pathologic studies,[6-8] and MC disease per se has not previously been reported in association with uterine cancer. However, some have suggested that MC-targeted treatment might be a helpful adjunct.[6,7]
Some of our patient’s symptoms that emerged during TAP therapy may have been due to chemotherapy. However, the full range of her symptoms (some of which long preceded the emergence of her cancer) was poorly explained by her cancer, other comorbidities, or cancer therapies, and some of them instead were potentially consistent with MC activation. The normal serum tryptase level suggested that SM was unlikely, and no increase in MCs was seen on pathologic re-examination of her tumor (Figure). Other MC mediator tests, however, confirmed MC activation, and her symptoms-many of which are classic symptoms of MC disease (eg, flushing, pruritus, vasomotor instability)-responded well to MCAS-targeted therapy, reappeared when she was off such therapy, and responded again to resumption of therapy.
Her 2+ years of post-treatment survival and good control of an ordinarily aggressive cancer are unusual and not likely due exclusively to cancer treatment. While the decreases in size and FDG avidity of her (presumably metastatic) adenopathy might be from chemotherapy, the timing of these improvements was poorly correlated with her chemotherapy, with some worsening of adenopathy occurring well into the second line of chemotherapy, and improvements in adenopathy continuing months after her last chemotherapy treatment. Waxing/waning adenopathy is a feature of MC disease.[9] It is possible that the patient’s adenopathy was a mixture of tumor and MCAS-driven inflammation, and that a combination of chemotherapy and MCAS-directed therapy produced the complicated, overall improved response seen over time. Also, some of her other inflammatory and fibrotic issues may have been partly driven by acute and chronic effects of her MCAS. Of hematopoietic origin, MCs are present in every human tissue but preferentially site themselves at environmental interfaces to better perform their principal function as sentinels against environmental insults.[10,11]
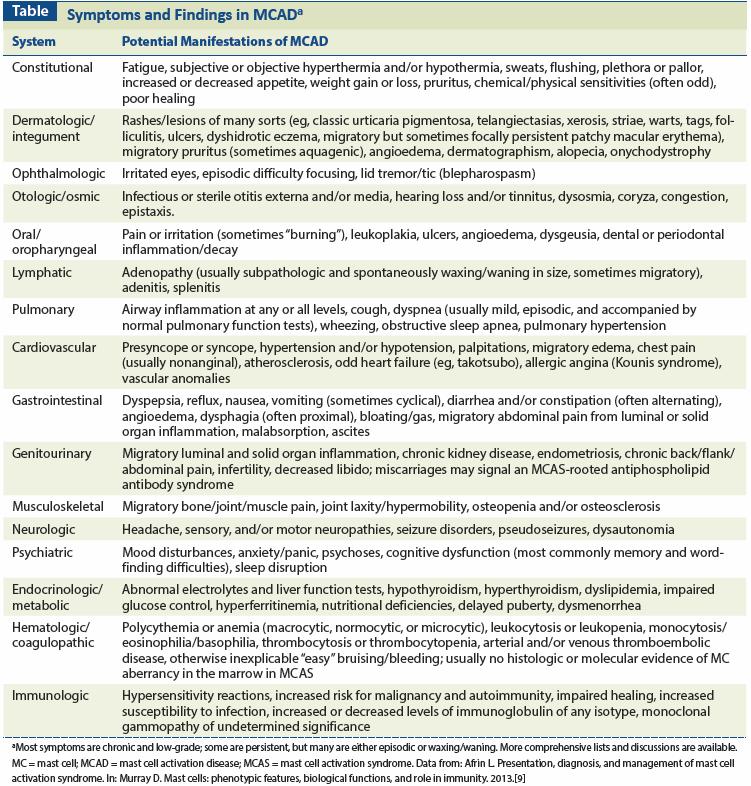
There are more than 200 MC mediators, each with a unique array of direct and indirect, local and remote effects.[12] MCAD-the new umbrella term for MC disease, encompassing both the long-recognized but rare mastocytosis[13-15] and the recently recognized and possibly quite prevalent MCAS[15,16]-often presents with chronic multisystem polymorbidity of a generally inflammatory nature,[1,9] and this can aggravate the risk and course of cancer.[17] MCAD often contributes to unusual presentations and courses of comorbidities. For example, it is possible that our patient’s MCAD may have contributed to her tumor’s microsatellite instability, rarely seen in UPSC.[18] In spite of its suspected prevalence, the recent recognition of MCAS (first cases published in 2007[19-21]) and its heterogeneous, multisystem presentations (Table)-often masked by more flagrant clinical problems, as in our patient-pose significant diagnostic challenges.
Slightly varying systems of diagnostic criteria for MCAS have been proposed by different groups,[1,22,23] and include a history consistent with aberrant MC mediator release; the presence of elevated MC markers in blood or urine; response to empiric MC-directed therapy; and an absence of other known causes of MC activation, such as SM, allergy, or physical urticaria. A detailed guide to the diagnosis of MCAD has recently been published.[24]
In contrast to cytoproliferative mastocytosis, MCAS is a relatively nonproliferative disease, and since the serum tryptase level is now recognized as reflecting total body MC load far more than total body MC activation state, it is elevated little to none in MCAS, in contrast to significantly elevated levels in SM.[1,23] Symptoms of aberrant MC mediator release are present in SM and MCAS, but only SM harbors aggregates of MCs in marrow or other extracutaneous tissue. Different presentations of MCAS may reflect different aberrant mediator expression patterns resulting from different constitutively activating mutations in the dominant MC regulatory element, Kit,[21,25] and other MC regulatory genes/proteins.[26,27]
Despite histories of chronic multisystem polymorbidity, often spanning decades, MCAS patients can benefit significantly from MC-directed therapies, including inhibitors of mediator production (eg, aspirin), mediator antagonists (eg, histamine and leukotriene receptor antagonists), and MC stabilizers (eg, cromolyn). Histamine H1 and H2 receptor antagonists and aspirin rapidly effected complete relief of all symptoms in our patient, and near-complete radiographic and biomarker remission emerged, which has been sustained now for 24 months. Nonetheless, optimal individualized therapy is not presently predictable (eg, based on symptoms), so persistence is required in stepping through available treatments.
Because our patient appears to be the first reported case of MCAS-associated malignancy, it is unknown whether her favorable outcome might be realized in similar patients with UPSC or other malignancies. Such a scenario, though, is not impossible to imagine, given that improved outcomes are seen when SM, comorbid with other hematologic malignancies, is recognized and treated,[28] as expected generally with control of inflammation in cancer.
This case suggests that comorbid MCAS can aggravate UPSC, and that control of MCAS may improve the course and outcome of metastatic UPSC. MCAS should be considered when UPSC (or, possibly, another malignancy) is present with other comorbidities and symptoms better explained by MC mediator release than by cancer.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Molderings GJ, Brettner S, Homann J, Afrin LB. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011;4:10.
2. Kosary C. Cancer of the corpus uteri. In: SEER survival monograph: cancer survival among adults: US SEER Program, 1988–2001, patient and tumor characteristics. Bethesda, MD: National Cancer Institute, SEER Program, NIH Pub; 2007.
3. Piura B, Meirovitz M, Shmulman M, et al. Uterine papillary serous carcinoma: study of 19 cases. Eur J Obstet Gynecol Reprod Biol. 1998;79:69-73.
4. Kitchener HC, Trimble EL; Endometrial Cancer Working Group of the Gynecologic Cancer Intergroup. Endometrial cancer state of the science meeting. Int J Gynecol Cancer. 2009;19:134-40.
5. Ren Y, Wang H, Zhou X, et al. Clinicopathological characteristics and HER-2/neu status in Chinese patients with uterine papillary serous carcinoma. ISRN Obstet Gynecol. 2011;2011:575327.
6. Ribatti D, Finato N, Crivellato E, et al. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193:1961-5.
7. Cinel L, Aban M, Basturk M, et al. The association of mast cell density with myometrial invasion in endometrial carcinoma: a preliminary report. Pathol Res Pract. 2009;205:255-8.
8. Erol YA, Ãzdemir Ã. Mast cells: Are they really related to invasiveness of endometrial carcinoma? Pathol Res Pract. 2010;206:426-7.
9. Afrin L. Presentation, diagnosis, and management of mast cell activation syndrome. In: Murray D, editor. Mast cells: phenotypic features, biological functions, and role in immunity. Hauppauge, NY: Nova Science Publishers; 2013. p. 155-232.
10. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215-23.
11. Akin C, Metcalfe DD. The biology of Kit in disease and the application of pharmacogenetics. J Allergy Clin Immunol. 2004;114:13-9.
12. Ibelgaufts H. Mast cells. COPE: Cytokines & cells online pathfinder encyclopedia. Available at: http://www.copewithcytokines.de/cope.cgi?key=mast%20cells.
13. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-61.
14. Rosbotham JL, Malik NM, Syrris P, et al. Lack of c-kit mutation in familial urticaria pigmentosa. Br J Dermatol. 1999;140:849-52.
15. Haenisch B, Nöthen MM, Molderings GJ. Systemic mast cell activation disease: the role of molecular genetic alterations in pathogenesis, heritability and diagnostics. Immunology. 2012;137:197-205.
16. Molderings GJ, Haenisch B, Bogdanow M, et al. Familial occurrence of systemic mast cell activation disease. PLoS ONE. 2013;8:e76241.
17. Galinsky DS, Nechushtan H. Mast cells and cancer-no longer just basic science. Crit Rev Oncol Hematol. 2008;68:115-30.
18. Gadducci A, Cosio S, Genazzani AR. Old and new perspectives in the pharmacological treatment of advanced or recurrent endometrial cancer: Hormonal therapy, chemotherapy and molecularly targeted therapies. Crit Rev Oncol Hematol. 2006;58:242-56.
19. Sonneck K, Florian S, Müllauer L, et al. Diagnostic and subdiagnostic accumulation of mast cells in the bone marrow of patients with anaphylaxis: monoclonal mast cell activation syndrome. Int Arch Allergy Immunol. 2007;142:158-64.
20. Akin C, Scott LM, Kocabas CN, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110:2331-3.
21. Molderings GJ, Kolck UW, Scheurlen C, et al. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42:1045-53.
22. Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126:1099-104.e4.
23. Valent P, Akin C, Arock M, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215-25.
24. Afrin LB, Molderings GJ. A concise, practical guide to diagnostic assessment for mast cell activation disease. World J Hematol. 2014. In press.
25. Molderings GJ, Meis K, Kolck UW, et al. Comparative analysis of mutation of tyrosine kinase kit in mast cells from patients with systemic mast cell activation syndrome and healthy subjects. Immunogenetics. 2010;62:721-7.
26. Kralovics R. Genetic complexity of myeloproliferative neoplasms. Leukemia. 2008;22:1841-8.
27. Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460-6.
28. Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116:5812-7.
