Incorporate CTCs into AJCC staging of metastatic breast ca?
Circulating tumor cells (CTCs) can help stratify patients with metastatic breast cancer in terms of likely survival and should therefore be considered for incorporation into the current AJCC staging system, according to a study presented at the 2007 San Antonio Breast Cancer Symposium (abstract 111).
SAN ANTONIO-Circulating tumor cells (CTCs) can help stratify patients with metastatic breast cancer in terms of likely survival and should therefore be considered for incorporation into the current AJCC staging system, according to a study presented at the 2007 San Antonio Breast Cancer Symposium (abstract 111).
Lead author Shaheenah Dawood, MD, of The University of Texas M.D. Anderson Cancer Center, explained that the research team had previously established that patients with more CTCs have poorer clinical outcomes (N Engl J Med 351:781-791, 2004) and that CTCs perform as well as or better than conventional risk factors in estimating prognosis (Clin Breast Cancer 7:471-479, 2007).
The new study therefore had two aims, she told ONI: “First, we wanted to confirm the prognostic role of CTCs in a larger cohort of women with metastatic breast cancer. Second, using these results, we wanted to propose a new stratification system for patients with metastatic breast cancer that could be incorporated into the AJCC [American Joint Committee on Cancer] staging system.”
Retrospective cohort study
The retrospective cohort study included 312 patients with metastatic breast cancer who were treated at M.D. Anderson between 2001 and 2007. A 7.5-mL blood sample was obtained from each patient at baseline and before the start of a first or new treatment. CTCs were isolated by an immunomagnetic assay (CellSearch, Veridex, LLC).
Patients’ CTC status was classified as negative (< 5 CTCs in the sample) or positive (≥ 5 CTCs).
Overall, the majority (66%) of the cohort had not received any prior treatment in terms of chemotherapy. Some 58% had bone metastases as their first site of metastatic disease, while 40% initially had visceral metastases; 60% had hormone- receptor-positive disease.
Overall survival
Median overall survival was 20.2 months for the entire cohort. Some 36% of the patients were CTC positive, and this group had significantly poorer median overall survival than their CTC-negative counterparts (13.5 vs 28.0 months).
Dr. Dawood noted that in multivariate analyses adjusted for hormone-receptor status, HER2 status, treatment status, and site of first metastasis, CTC-positive patients still had an elevated risk of death (hazard ratio 3.06).
Among the 28% of patients with newly diagnosed and untreated metastases, the CTC-positive subset had significantly poorer overall survival than the CTC-negative subset (15 vs 29.3 months) (see Figure).
Thus, baseline CTC status in patients with newly diagnosed metastatic breast cancer should be considered for inclusion in the revision of the AJCC staging system for stage IV breast cancer, Dr. Dawood commented.
“These data confirm the heterogeneity of metastatic breast cancer. Stratification of patients and evaluation of clinical benefit based on detection and monitoring of CTCs should be advocated and incorporated into clinical trials,” Dr. Dawood concluded.
She said that the research team is seeking confirmation of early findings that the change in CTC levels can serve as an indicator of the efficacy of treatment.
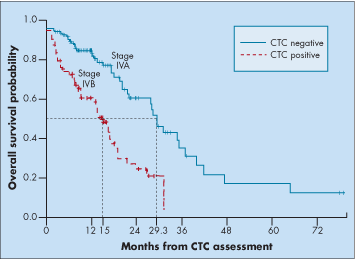
Prognostic stratifi cation of patients with newly diagnosed, untreated metastatic breast cancer into two subgroups based on CTC status: stage IVA (CTC negative) and stage IVB (CTC positive).