Integrins and Cancer
Integrins play an important physiologic role in cell adhesion, and accumulating evidence suggests that they also regulate cell growth, proliferation, migration, and apoptosis. A number of congenital and acquired disease states have been associated with integrins, and small- molecule integrin inhibitors have been approved for treatment of benign hematologic diseases. In cancer, aberrant expression with normal functioning rather than dominant genetic variations of genes coding for integrins has generally been observed. This aberrant expression is mediated through "bidirectional" receptor signaling and interaction with corresponding signals from growth factor signaling pathways, leading to inhibition of apoptosis, induction of cell proliferation, extracellular matrix remodeling, migration, and angiogenesis. From a clinical perspective, a growing number of molecules targeting integrins have been developed for treatment and imaging purposes; clinical studies in melanoma, prostate cancer, and other malignancies are underway. This review summarizes the biology of integrins, the signal transduction pathways they regulate, and their role in different stages of carcinogenesis. Furthermore, it provides a synopsis on the clinical advancements in integrin targeting for therapeutic and imaging purposes in cancer.
ABSTRACT: Integrins play an important physiologic role in cell adhesion, and accumulating evidence suggests that they also regulate cell growth, proliferation, migration, and apoptosis. A number of congenital and acquired disease states have been associated with integrins, and small- molecule integrin inhibitors have been approved for treatment of benign hematologic diseases. In cancer, aberrant expression with normal functioning rather than dominant genetic variations of genes coding for integrins has generally been observed. This aberrant expression is mediated through "bidirectional" receptor signaling and interaction with corresponding signals from growth factor signaling pathways, leading to inhibition of apoptosis, induction of cell proliferation, extracellular matrix remodeling, migration, and angiogenesis. From a clinical perspective, a growing number of molecules targeting integrins have been developed for treatment and imaging purposes; clinical studies in melanoma, prostate cancer, and other malignancies are underway. This review summarizes the biology of integrins, the signal transduction pathways they regulate, and their role in different stages of carcinogenesis. Furthermore, it provides a synopsis on the clinical advancements in integrin targeting for therapeutic and imaging purposes in cancer.
TABLE 1

The Four Families of Cell Adhesion Molecules
Throughout its life, a cell must constantly sense physicochemical changes in the microenvironment and respond by making critical decisions regarding the processes of proliferation, division, motility, life, and death. In multicellular organisms different cell types coordinate functions to facilitate tissue homeostasis by exchanging signals, such as growth factors or other cyto-kines, and by secreting, assembling, and remodeling an insoluble network of proteins termed the extracellular matrix (ECM). The ECM provides a structural framework that enables cells either to anchor for stationary existence, or to migrate. It also influences cell size, shape, and interaction with other cells in tissue formation. Cell adhesion to the ECM is predominantly mediated by integrins, the most structurally and functionally diverse family of cell adhesion molecules, which regulate cell-cell and cell-ECM interactions (Table 1). Extensive research over the past 2 decades has shown that integrins not only "glue" cells to the ECM, but also regulate important signal transduction pathways for cell growth, proliferation, migration, or apoptosis.
TABLE 2
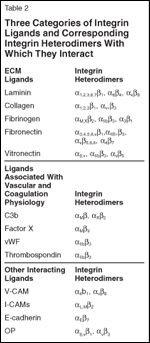
Three Categories of Integrin Ligands and Corresponding Integrin Heterodimers With Which They Contact
The significance of integrins in cellular physiology and body homeostasis is reflected by an increasing number of disease states, ranging from hematologic (leukocyte adhesion deficiency type I, Glanzmann's thrombasthenia, hypercoagulable states pertaining to cardiovascular and cerebrovascular diseases), dermatologic (epidermolysis bullosa, oral pemphigoid), and musculoskeletal (congenital myopathy) that have been associated with altered expression or activation of integrins (see Reference 1 and references therein). Moreover, small-molecule integrin inhibitors have already been approved by the US Food and Drug Administration (FDA) for treatment of acute coronary syndrome.
In the cancer setting, interest has been fueled by recent advances in our understanding that integrins are upregulated and/or activated in several human malignancies that are critical components of almost all critical phases of tumorigenesis, that they interact with major oncogenic signal transduction pathways, and that they regulate important cancer-related phenomena, such as migration-invasion, metastasis, and angiogenesis. This review summarizes the role of integrins in cancer, and provides preliminary data regarding the development of integrin antagonists for therapeutic and imaging purposes.
Integrin Structure and Biology
Understanding the molecular biology of integrins is important in order to explain their role in cancer. Integrins are large glycoproteins assembled from a set of different, non–covalently associated type I transmembrane α- and β-subunits leading to 24 differentially composed heterodimers with redundant but also specific ligand binding (Figure 1). These different heterodimers make integrins the most diverse family of cell adhesion molecules, accounting for the signaling differences and interactions with different binding ligands (Table 2). A schematic representation of integrin signaling is shown in Figure 2.
FIGURE 1
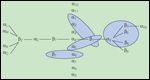
The 24 Possible αβ Integrin Heterodimers
Each subunit has multiple domains (reviewed in Reference 2 and references therein) that mediate large conformational allosteric changes between high-energy barrier states in response to ligand binding such that a low-affinity, closed, unliganded "bent" conformation transitions to the high-affinity, open-liganded, "extended" conformation by a "switchbladelike" motion during which separation of the integrin cytoplasmic domains "legs" occurs. The equilibrium between the "bent" and the "extended" conformation is affected by the relative concentration of extracellular ligands and intracellular signaling molecules, such as protein-kinase C or phosphatidylinositol phosphate kinase type-γ (PIPKγ). These signaling molecules may activate talin, which interacts with the cytoplasmic domains of the β-subunit and primes the high-affinity unliganded "extended" conformation that exposes the ligand binding site.
Integrin Signaling
FIGURE 2
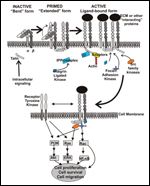
Integrin Signaling
Thus, integrins are the prime example of bidirectional receptor signaling: "Inside-out" signals induce conformational changes between the integrin "headpiece" and the cytoplasmic domains with exposure of neoepitopes, termed ligand-induced binding sites (LIBS), and acquisition of high-affinity states necessary for binding of "outside-in" signals that regulate cellular responses (reviewed in Reference 3). This bidirectional signaling is very important in cancer because constitutive activation of integrins from endogenous stimuli mediates stronger binding to the ECM and therefore a more dynamic interaction of these adhesion receptors with their substrates, which is necessary for migration and metastasis.
Several integrins, such as αIIbβ3, αvβ3, and α5β1, recognize the RGD (Arg-Gly-Asp) sequence in their matrix ligands, and a family of integrin ligands containing RGD motifs, termed disintegrins, can inhibit integrin function. However, other specific recognition sequences have been described, such as the LDV motif for α4β1 or the IDS sequence for VCAM-1 further adding to the functional diversity of integrin ligands.
The role of genetic variations in the genes coding integrins and cancer risk is only now beginning to be explored. A few polymorphisms in the α or β integrin subunits have been correlated with increased breast cancer risk[4] or metastatic potential in renal cell carcinoma[5] by enhancing "outside-in" signaling. Moreover, a point mutation in the β1 integrin subunit of squamous cell carcinoma of the tongue is the first mutation described in cancer that results in constitutive activation of ligand binding.[6] Other population studies, however, have not suggested any genetic variations, implying that it may be the aberrant expression, albeit with normal function, that is the mechanism by which integrins play a role in cancer.
Unlike growth factor receptors, integrins lack intrinsic tyrosine kinase activity. Upon ligand binding, integrins crosslink and cluster, leading to the formation of cellular membrane structures termed focal adhesions that are the point of convergence between ECM components and the intracellular cytoskeleton. A diverse number of structural and signaling proteins are known to be located at these sites. Upon integrin aggregation by ligand binding, talin interacts with the integrin β-subunit and recruits FAK (focal adhesion kinase; FAK is a nonreceptor tyrosine kinase that oligomerizes at focal adhesions and is activated by trans-autophosphorylation at residue Tyr397. Autophosphorylated FAK thus functions as a phosphorylation-regulated "scaffold" that recruits SFKs (Src-family kinases) and activates PI3K (phosphatidylinositol 3-kinase) and ERK (extracellular signal-regulated kinase)/MAPK (mitogen-activated protein kinase).
With these interactions integrins activate a number of signal transduction pathways, such as the Raf-ERK/MAPK, PI3K-Akt, nuclear factor–kappa B (NF-κB), and Jun which may assist in induction of cell proliferation and protection from apoptosis. The activation of these pathways by SFKs and/or FAK occurs by activation and recruitment of several adaptor proteins that are either specifically involved in cytoskeleton-dependent signaling networks, such as p130Cas, paxillin and Crk-DOCK180, or shared with other growth factor signaling pathways, such as the Grb2-mSOS complex (reviewed in Reference 7 and references therein).
Integrins are prime regulators of adhesion, spreading, and migration.[8] Monovalent ligand binding to an extracellular ligand is not sufficient to induce integrin clustering and subsequent complex formation. The transition from the integrin activation to the clustering phase is a poorly understood phenomenon[9] in which members of the Rho family of proteins are involved. These proteins cycle between an active GTP-bound state and an inactive GDP-bound state under the control of guanine exchange factors. Thus, integrin binding initially activates the Rho proteins, Rac1 or Cdc42, and actin polymerization occurs. Subsequently, RhoA becomes active and phosphorylates myosin light chains, an event that allows for binding of myosin to actin filaments and therefore increased contractility.
Cell migration is controlled by differential integrin dynamics and differential activity of Rho proteins at the actin cytoskeleton–ECM junction. At the cell front, Rac1 or Cdc42 activity predominates and induces formation of actin-rich, low-density, slow-turnover focal complexes that consist of loose low-tension F-actin, high-stability integrin clusters. At the cell rear, however, RhoA activity predominates and induces formation of high-density, fast-turnover focal contacts, consisting of high-tension, stress fibers.[1]
It is obvious that several molecules which mediate integrin signaling also participate in signaling events initiated by soluble growth factors and their transmembrane receptors; in fact, integrins are necessary for optimal activation of growth factor receptors. For example, integrins sustain the rather transient growth factor–mediated activation of ERKs,[10] and sustain upregulation of cyclin D1 transcription and suppression of cyclin-dependent kinase inhibitors.[11] Moreover, adhesion-dependent but ligand-independent activation of growth factor receptors has been reported.[12] The mechanisms underlying this cooperative signaling may range from direct physical interaction between integrins with growth factor receptors[13] to indirect interactions through mutual adaptor proteins that may interact either with the growth factor receptor or any of its downstream effectors.[12]
Integrin signaling is regulated by a complex heterogeneous class of proteins, the most important of which are the integrin-linked kinases (ILK), and the adaptor family of proteins PINCH (particularly interesting Cys-His–rich proteins) and parvin reviewed in Reference 14. During steady-state condition these proteins are in a preformed complex within the cytoplasm, termed the IPP (ILK-PINCH-parvin) complex. Upon integrin activation the IPP complex is recruited to focal adhesions through interaction with other factors, such as paxillin. Within the focal adhesions, the IPP complex bridges with F-actin through parvin and the actin-binding adaptor molecule vinculin. The interaction of the IPP complex with different signal transduction pathways is regulated by the particular PINCH isoform (1 or 2) and parvin protein (α-, β-, or γ-) which is loaded onto the IPP complex. The activity of ILK is positively regulated in a PI3K signaling matter, and by adhesion to the ECM or by growth factors and other chemokines, whereas it is negatively regulated by the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and protein phosphatase 2C (PP2C). In normal cells, ILK is transiently activated and can influence a diverse set of signal pathways and cell functions, such as migration, motility, invasion, proliferation, and angiogenesis.
Role in Tumorigenesis
Alterations in integrin signaling are involved in nearly all steps of carcinogenesis, ranging from switches in the utilization of αβ heterodimers, to aberrant expression of integrins, and constitutive activation of downstream effectors of integrin signaling and interactions with other signaling pathways. Below we summarize the most recent advances in understanding the role of integrins in cancer.
Invasion
In order for cells to acquire an invasive phenotype they have to disrupt their interactions with their normal neighboring cells, lose their apical-basal polarity (if epithelial), remodel their cytoskeleton, and acquire the ability to migrate. Constitutive high expression and/or activation of SFKs is a frequent phenomenon in early stages of carcinogenesis and regulates the mesenchymal transition in epithelial[15] and nonepithelial malignancies.[16] Elevated Src activity disrupts the function of cadherins important for homotypic (E-cadherin) and heterotypic (N-cadherin) cell-cell adhesions, assists in translocation of β-catenin to the nucleus where it activates genes involved in the invasive tumor phenotype, and activates FAK, thereby assisting in assembly of dynamic integrin-mediated focal adhesion–like structures with the ECM. Moreover, the signals from the microenvironment, such as the transforming growth factor–beta (TGF-β), upregulates αvβ6, a receptor for fibronectin.[17]
Acquisition of Transformed Phenotype
Integrins play a significant role in the acquisition and maintenance of neoplastic phenotype by preventing apoptosis and maintaining cell proliferation. Integrin expression profile dramatically changes upon the normal-to-neoplastic transition.[18] In normal epidermis the most abundant integrins are α2β1 (collagen receptor), α3β1 (laminin), and α6β4(laminin), whereas α5β1 (fibronectin), αvβ5 (vitronectin), and αvβ6 (fibronectin/tenascin) are expressed at low or undetectable levels.[19,20] The signaling of α6β4integrin is necessary to support the receptor tyrosine kinase ErbB2-mediated tumor cell proliferation and inhibition of apoptosis by activating c-Jun and STAT3[21] and, vice versa, increased ErbB2 signaling stimulates adhesiveness to fibronectin and therefore survival via α5β1 upregulation in MCF-7 breast cancer cells.[22] These changes may reflect selective pressure imposed upon cancer cells to free themselves from adhesion to basement membranes and to upregulate integrins that foster survival and proliferation.
There is increasing evidence that integrin signaling is upregulated in cancer. Upregulation of FAK and ILK is a frequent phenomenon in a variety of human cancers.[23] Constitutive activation of FAK abolishes the requirement for adhesion of cells to the ECM and prevents apoptosis secondary to inadequate or inappropriate cell substrate contact ("anoikis").[24] Similarly, ILK is also overexpressed in several cancers,[25] and by activating several signal transduction pathways confers cancer cell growth and antiapoptotic advantage. Increased integrin signaling is attributed to both integrin binding to ECM ("outside-in" signaling) and intracellular mechanisms which in turn modulate the binding properties of the receptor ("inside-out" signaling).[26]
Cross-talk between integrins and growth factor receptors is an equally important mechanism which modulates the functional activity of integrins. For example, integrins may aberrantly respond to growth factors, as in the case of heregulin, a growth factor associated with breast cancer aggressiveness which activates ERK1/ERK2 signaling only through αvβ3.[27]
Migration
Control of cell migration depends on cytoskeletal rearrangements and an ordered redistribution of integrin molecules at the cell surface, as cells attach and detach from the ECM while they move forward and constantly change their shape. This rapid sequence of events is facilitated by the Rho GTPases, which are frequently overexpressed in many human cancers. In contrast with the Ras family of GTPases, in which activating point mutations result in constitutive binding of GTP and therefore increased downstream signaling pathways are known, no such mutations have been described for the Rho GTPases (reviewed in Reference 28). The Rho GTPases Rho, Rac1, and Cdc42, however, have been shown to participate in the earlier stages of tumorigenesis, such as loss of epithelial cell polarity, disruption of basal membrane, and increased proliferation, by stimulating motility and ECM degradation. Cancer cell motility is regulated by certain integrins[29,30] via certain signal transduction pathways[31,32] common to cell survival and proliferation.
Extracellular Matrix Remodeling
Normal stroma can delay or prevent tumorigenesis, whereas abnormal stroma that is loose and edematous at the expanding tumor fronts, but dense in central tumor areas, can promote tumor growth. Cancer cells are able to modulate their microenvironment by inducing partial degradation of the ECM and producing abnormal stroma via an incompletely understood process termed "stromatogenesis."[33] This remodeling process shares several similarities with wound healing in that normal bystanders, such as macrophages and fibroblasts, assist in this degradation-regeneration process.
In the case of carcinogenesis, however, they receive erroneous activating growth factor signals by neighboring cancer cells. They secrete matrix metalloproteases (MMPs), a family of endopeptidases secreted as zymogens, which are capable of degrading most components of the basal membrane and ECM. Activation of several integrins may result in increased expression[34] or enhanced maturation of MMPs.[35] The increased interstitial pressure and matrix rigidity upregulates integrin expression and results in increased Rho-mediated cytoskeletal tension, formation of dense focal contacts and activation of growth factor–dependent signal transduction pathways in a positive feedback loop.[36] This implies that alteration in the mechanical properties of ECM has consequences not only for cellular shape, but also for the proliferative capacity of cancer cells.
Metastasis
In order to colonize distant target organs, cancer cells must detach from the primary tumor, gain access to blood vessels and survive within the vasculature exposed to shear forces which physically oppose cell attachment. Activated αvβ3 may rescue blood circulating cancer cells from shear-induced tumor cell arrest by binding to leukocytes and platelets to survive-much like the "leukocyte" integrins of the α4 and β2 families or the "platelet" integrin αIIbβ3[37]. Once localized to the metastatic environment cancer cells of different tissue origins may utilize distinct integrin-ligand combinations to colonize the same target organ and receive local mitogenic stimuli. For example, in most cancers the αvβ3 integrin is the prime homing ligand to support adhesion and migration to bone matrix.
Moreover, overexpression of all β3 integrins, N-cadherin and MCAM in a melanoma tissue microarray was associated with a higher incidence of worse prognosis gastrointestinal metastasis (AJCC M1c) rather than development of more favorable subcutaneous and lymph node metastasis (AJCC M1a, M1b).[20] In their activated state, several integrins recognize ligands which are usually not bound when unactive. Such aberrant binding may favor colonization of certain microenvironments, such as the bone matrix via sialoprotein or interact with molecules which further enhance malignant behavior, such as osteopontin. Osteopontin is a secreted glycoprotein that is overexpressed in a number of different carcinomas and can promote adhesion, migration and metastasis by binding predominantly to the αvβ3 integrin and the CD44 antigen.[38] Thus, more aggressive cancers not only tend to express more osteopontin but also may be more responsive to this protein.
Angiogenesis
Integrins play an important role in the normal functioning of tumor vasculature. The morphologic and metabolic diversity of endothelial cells is reflected on the differential expression pattern of integrin receptors between the quiescent established blood vessels and the proliferating angiogenic blood vessels, which, in addition, express the αvβ3, αvβ5, and α5β1 integrins. A variety of pharmacological manipulations of αvβ3/αvβ5 integrins in vitro and in vivo models have suggested that αvβ3/αvβ5 blockade selectively inhibits neoangiogenesis whereas quiescent and preexisting vessels are not perturbed.[39] These results have been challenged by recent reports showing that mice either lacking all five αv-containing integrins or lacking β3 and/or β5 integrins are viable but unable to undergo extensive developmental vasculogenesis. They also displayed enhanced postnatal angiogenesis in response to hypoxia, VEGF, and tumor grafting.[40,41] Activation of αvβ3, α6β4and/or their downstream integrin effectors stimulate vascular endothelial growth factor (VEGF) expression, the most potent angiogenic molecule,[42-45] and may promote activation of the receptor for VEGF by direct physical interaction between β3 integrin and VEGFR2.[46] Interestingly, VEGF can, in turn, activate αvβ3 and therefore increase adhesion of endothelial cells to a variety of ligands.[47] VEGF-independent mechanisms accounting for the angiogeneic effect of αvβ3 have also been described.[48]
However, expression of VEGFR2 is elevated in β3-deficient mice and several natural ligands of αvβ3, such as thrombospondins, tumstatin, and proteolytic products of MMP-2 and collagen IV α3 chain are known inhibitors of antiogenesis.[40] These discordant results may reflect differences between developmental vs postnatal angiogenesis, and that missing molecules, such as αvβ3, may elicit compensatory changes by other integrins. Accumulating evidence suggests that other intergrins may have an equally important role, such as α6β4,[49] α1β1/α2β1,[50] and α5β1.[51]
Cancer Therapeutics Targeting the Integrins
The undisputed role of integrins in tumorigenesis has raised significant interest in application of cancer therapeutics. A number of molecules ranging from β1 and β3 integrin antagonists to inhibitors of downstream effects of integrin signaling, such as MMPs, FAK, ILK are at different stages in drug development.[52] Currently, attention is focused on development of αv (β3 and β5) and α5β1 antagonists, which are particularly associated with tumor angiogenesis. For now, these inhibitors bind to the extracellular part of the integrin taking into consideration the RGD sequence in several integrins. Thus, several integrin inhibitors are small molecule antagonists, which function as ligand- mimetic competitive inhibitors (peptidomimetics),[53] whereas others, such as the antagonistic antibody LM-609, recognize a conformational epitope on the αvβ3 integrin distinct from the RGD recognition site. In many circumstances integrin antagonists may induce LIBS epitopes or even the active conformation of integrins and, therefore, have a rather partial agonist effect.
LM-609 is a mouse monoclonal antibody specific for αvβ3 that has been shown to block angiogenesis by interrupting the adhesion of αvβ3 to its physiologic ligands.[39] Through a combinatorial mutagenesis strategy LM-609 was converted to a fully humanized antibody, called Vitaxin. Proof-of-concept effects of Vitaxin in decreasing blood flow through tumor vasculature was shown in a phase I trial in advanced solid tumors.[54] In this as well as other phase I clinical trials of Vitaxin and other integrin inhibitors, safety was confirmed although the best objective response was only disease stability.[55-57] A small (n = 112) randomized phase II study of Vitaxin given alone or in combination with dacarbazine in patients with metastatic melanoma suggested that the disease-free survival of the combination treatment arm was inferior to single-agent Vitaxin administration,[58] implying that better understanding of other non-antiangiogenic mechanisms of action of these molecules is needed in order to rationally combine them with other agents.[20] Interestingly enough, however, and at the time of the study report, the disease free survival of either arm was longer than concurrent historic controls, suggesting that these patients may have done well without relapse because treatment prevented new metastases, and not because of tumor shrinkage.
Integrin Imaging in Cancer
The specificity of αvβ3 upregulation only for the angiogenic endothelial cells has stimulated great interest in developing αvβ3 selective tracers for noninvasive imaging of tumor angiogenesis. Apart from the obvious benefit of imaging tumor angiogenesis in a variety of different malignancies αvβ3-imaging takes into account the heterogeneity of αvβ3 expression in different tumors/stages and provides an opportunity to improve the therapeutic index of αvβ3-targeted therapies by selecting out only patients with high levels of αvβ3 expression in their tumor. A significant correlation between the staining intensity of microvessel density in the tumor biopsies and the standard uptake value by [18F]Galacto-RGD imaging of a variety of human malignancies proved the potential benefit of this imaging approach for individualized therapy with αvβ3 antagonists.[59]
Conclusions
Besides growth factors, angiogenesis, apoptosis, cell cycle, and tumor immunology which have attracted the most interest in understanding the molecular biology of cancer and therefore the development of modern targeted specific therapies, it bears no question that integrin biology is interwoven with each and every one of these processes and that its dysregulation is not only involved in each and every step of tumorigenesis, but critically affects cell cycle, apoptosis, and aggressiveness. Unsuccessful treatments targeting all these processes described above have to take into account the role of integrins in cancer and, vice versa, the present lack of efficacy in most integrin antagonists clinically tested so far, has to take into account their cross-talk with these processes. It is more likely that combinatorial therapeutics which considers redundant pathways may be more efficacious that single-agent treatments. Therefore further research in the integrin field may ultimately suggest more rational treatment combinations.
Financial Disclosure:Dr. Kirkwood has acted as a consultant for MedImmune.
References:
1. Wehrle-Haller B, Imhof BA: Integrin-
dependent pathologies. J Pathol 200(4):481-487, 2003.
2. Luo BH, Springer TA: Integrin structures and conformational signaling. Curr Opin Cell Biol 18(5):579-586, 2006 [e-pub Aug 14, 2006].
3. Ginsberg MH, Partridge A, Shattil SJ: Integrin regulation. Curr Opin Cell Biol 17(5):509-516, 2005.
4. Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG: Integrin beta3 Leu33Pro homozygosity and risk of cancer. J Natl Cancer Inst 95(15):1150-1157, 2003.
5. Kallio JP, Mikkelsson J, Tammela TL, et al: Genetic variation in platelet integrin alphabeta (GPIIb/IIIa) and the metastatic potential of renal cell carcinoma. BJU Int 98(1):201-204, 2006.
6. Evans RD, Perkins VC, Henry A, et al: A tumor-associated beta 1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol 160(4):589-596, 2003 [e-pub 2/10/03].
7. Giancotti FG, Ruoslahti E: Integrin signaling. Science 285(5430):1028-1032, 1999.
8. Wiesner S, Legate KR, Fassler R: Integrin-actin interactions. Cell Mol Life Sci 62(10):1081-1099, 2005.
9. Hsieh CF, Chang BJ, Pai CH, et al: Stepped changes of monovalent ligand-binding force during ligand-induced clustering of integrin alphaIIB beta3. J Biol Chem 281(35):25466-25474, 2006 [e-pub 6/21/06].
10. Assoian RK, Schwartz MA: Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev 11(1):48-53, 2001.
11. Bao W, Thullberg M, Zhang H, et al: Cell attachment to the extracellular matrix induces proteasomal degradation of p21(CIP1) via Cdc42/Rac1 signaling. Mol Cell Biol 22(13):4587-4597, 2002.
12. Moro L, Dolce L, Cabodi S, et al: Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 277(11):9405-9414, 2002 [e-pub 12/ 27/01].
13. Borges E, Jan Y, Ruoslahti E: Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem 275(51):39867-39873, 2000.
14. Legate KR, Montanez E, Kudlacek O, et al: ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol 7(1):20-31, 2006.
15. Avizienyte E, Fincham VJ, Brunton VG, et al: Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial-mesenchymal transition. Mol Biol Cell 15(6):2794-2803, 2004 [e-pub 4/9/04].
16. Qi J, Wang J, Romanyuk O, et al: Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell 17(3):1261-1272, 2006 [e-pub 12/21/05].
17. Bates RC, Bellovin DI, Brown C, et al: Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 115(2):339-347, 2005.
18. Plantefaber LC, Hynes RO: Changes in integrin receptors on oncogenically transformed cells. Cell 56(2):281-290, 1989.
19. Janes SM, Watt FM: Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol 166(3):419-431, 2004.
20. Watson-Hurst K, Becker D: The role of N-cadherin, MCAM and beta(3) integrin in melanoma progression, proliferation, migration and invasion. Cancer Biol Ther 5(10):1375-1382, 2006 [e-pub 10/26/06].
21. Guo W, Pylayeva Y, Pepe A, et al: Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126(3):489-502, 2006.
22. Spangenberg C, Lausch EU, Trost TM, et al: ERBB2-mediated transcriptional up-regulation of the alpha5beta1 integrin fibronectin receptor promotes tumor cell survival under adverse conditions. Cancer Res 66(7):3715-3725, 2006.
23. van Nimwegen MJ, van de Water B: Focal adhesion kinase: A potential target in cancer therapy. Biochem Pharmacol 73:597-609, 2006 [e-pub 9/25/06].
24. Duxbury MS, Ito H, Zinner MJ, et al: Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery 135(5):555-562, 2004.
25. Hannigan G, Troussard AA, Dedhar S: Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat Rev Cancer 5(1):51-63, 2005.
26. Vellon L, Menendez JA, Lupu R: A bidirectional "alpha(v)beta(3) integrin-ERK1/ERK2 MAPK" connection regulates the proliferation of breast cancer cells. Mol Carcinog 45(10):795-804, 2006.
27. Vellon L, Menendez JA, Lupu R: AlphaVbeta3 integrin regulates heregulin (HRG)-induced cell proliferation and survival in breast cancer. Oncogene 24(23):3759-3773, 2005.
28. Gomez del Pulgar T, Benitah SA, Valeron PF, et al: Rho GTPase expression in tumouri-genesis: evidence for a significant link. Bioessays 27(6):602-613, 2005.
29. Zhou H, Kramer RH: Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J Biol Chem 280(11):10624-10635, 2005 [e-pub 12/17/04].
30. Chen M, O'Connor KL: Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 24(32):5125-5130, 2005.
31. Vial E, Sahai E, Marshall CJ: ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4(1):67-79, 2003.
32. Keely PJ, Westwick JK, Whitehead IP, et al: Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 390(6660):632-636, 1997.
33. Sivridis E, Giatromanolaki A, Koukourakis MI: "Stromatogenesis" and tumor progression. Int J Surg Pathol 12(1):1-9, 2004.
34. Hu B, Jarzynka MJ, Guo P, et al: Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res 66(2):775-783, 2006.
35. Rolli M, Fransvea E, Pilch J, et al: Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA 100(16):9482-9487, 2003 [e-pub 6/ 21/03].
36. Paszek MJ, Zahir N, Johnson KR, et al: Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241-254, 2005.
37. Felding-Habermann B, O'Toole TE, Smith JW, et al: Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA 98(4):1853-1858, 2001.
38. Samanna V, Wei H, Ego-Osuala D, et al: Alpha-V-dependent outside-in signaling is required for the regulation of CD44 surface expression, MMP-2 secretion, and cell migration by osteopontin in human melanoma cells. Exp Cell Res 312:2214-2230, 2006 [e-pub 4/24/06]
39. Brooks PC, Montgomery AM, Rosenfeld M, et al: Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79(7):1157-1164, 1994.
40. Reynolds LE, Wyder L, Lively JC, et al: Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 8(1):27-34, 2002.
41. Bader BL, Rayburn H, Crowley D, et al: Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 95(4):507-519, 1998.
42. De S, Razorenova O, McCabe NP, et al: VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci USA 102(21):7589-7594, 2005 [e-pub 5/16/05].
43. Lipscomb EA, Simpson KJ, Lyle SR, et al: The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res 65(23):10970-10976, 2005.
44. Tan C, Cruet-Hennequart S, Troussard A, et al: Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell 5(1):79-90, 2004.
45. Mitra SK, Mikolon D, Molina JE, et al: Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene 25:5969-5984, 2006 [e-pub 5/8/06].
46. Mahabeleshwar GH, Feng W, Phillips DR, et al: Integrin signaling is critical for pathological angiogenesis. J Exp Med 203(11):2495-2507, 2006.
47. Byzova TV, Goldman CK, Pampori N, et al: A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell 6(4):851-860, 2000.
48. Silletti S, Kessler T, Goldberg J, et al: Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci USA 98(1):119-124, 2001.
49. Nikolopoulos SN, Blaikie P, Yoshioka T, et al: Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 6(5):471-483, 2004.
50. Senger DR, Claffey KP, Benes JE, et al: Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci USA 94(25):13612-13617, 1997.
51. Francis SE, Goh KL, Hodivala-Dilke K, et al: Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 22(6):927-933, 2002.
52. Paulhe F, Manenti S, Ysebaert L, et al: Integrin function and signaling as pharmacological targets in cardiovascular diseases and in cancer. Curr Pharm Des 11(16):2119-2134, 2005.
53. Shimaoka M, Springer TA: Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov 2(9):703-716, 2003.
54. McNeel DG, Eickhoff J, Lee FT, et al: Phase I trial of a monoclonal antibody specific for alphavbeta3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res 11(21):7851-7860, 2005.
55. Eskens FA, Dumez H, Hoekstra R, et al: Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer 39(7):917-926, 2003.
56. Gutheil JC, Campbell TN, Pierce PR, et al: Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res 6(8):3056-3061, 2000.
57. Cianfrocca ME, Kimmel KA, Gallo J, et al: Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer 94(11):1621-1626, 2006.
58. Hersey P, Sosman J, O'Day S, et al: A phase II, randomized, open-label study evaluating te antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human alpha v beta 3 (avb3) integrin, +/- dacarbazine (DTIC) in patients with metastatic melanoma (MM) (abstract 7507). J Clin Oncol 23(suppl 16S):711s, 2005.
59. Beer AJ, Haubner R, Sarbia M, et al: Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res 12(13):3942-3949, 2006.