Investigators Pool Data to Better Classify Prognosis in Gastric Tumor
In a presentation at 2022 IGCC, Ju-Seog Lee, PhD, discussed an analysis that combined tumor classification models to derive new prognostic subgroups in gastric cancer.
Genomics data collected from various gastric cancer classification systems were analyzed to determine if consensus could be derived from the efforts of different investigative teams, according to a presentation at the 2022 International Gastric Cancer Conference.1
“Classification of tumors is key for the successful treatment of patients with the gastric tumors, [including] conventional staging such as TNM and WHO [World Health Organization] classification, which have been successfully used for that purpose,” Ju-Seog Lee, PhD, associate professor in the Department of Systems Biology, Division of Cancer Medicine at The Univesity of Texas MD Anderson Cancer Center in Houston, said during a presentation of the data. “However, there are some limitations because it does not reflect the underlying biology of the of the gastric tumor, which is a key for targeted treatment.”
Lee said that different groups have experimented with the use of genomics, proteomics, and metabolomics to define gastric subtypes—such as his own group that previously identified 2 distinct molecular subtypes called mesenchymal phenotype (MP) and epithelial phenotype (EP).2 Other signals of prognosis that his research team have explored include activation or inactivation of the Hippo pathway, presence of Epstein-Barr virus (EBV), microsatellite instability (MSI) status, and the number of somatic copy number alterations.3,4
“It doesn’t stop there. Many other investigators have also identified different subtypes of disease that are well associated with prognostic differences, and this is great progress,” Lee said. “However, it raised new and different questions how [are these systems] similar to each other and is there any consensus among these different classifications based on genomic approaches.”
Methods
To address the clinical question, the investigators collected genomic data for the 8 prognostic subgroups identified. These included:
- Histology by intrinsic subtype
- Hippo pathway expression3
- Four molecular subtypes identified by The Cancer Genome Atlas Project (TCGA4)4
- TCGA risk score (TRS)4
- Asian Cancer Research Group (ACRG) subtype5
- Yonsei Cancer Center subtype6
- LNC6 subtypes7
- MP2
In total, gene-expression profile data were available for 2527 samples, comprised of 1427 in the training set and 1100 in the validation set. Clinical data were available for each.
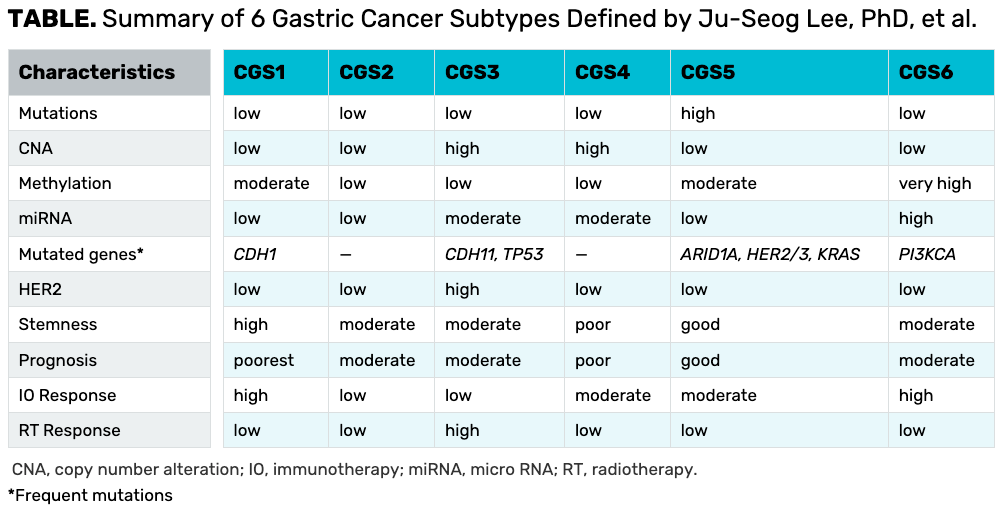
As part of the investigation, Lee and colleagues used the information from the 8 classifications to further analyze patients in a process known as cluster of clusters assignment to arrive at 6 new tumors subtypes. He pointed out that these new groups were derived from previously classifications rather than genomic data, with group 1 having the worst outcomes compared with groups 5 and 6 with the best outcomes.
Intergroup differences included a high mutational burden in subgroup 5, with highly mutated HER3 and HER4. The highest copy number alterations were noted in groups 3 and 4.
Real-World Validation
Lee turned to the phase 3 ARTIST trial (NCT00323830), which examined capecitabine plus cisplatin (XP) alone vs XP with radiotherapy (XP/RT) in patients with curatively resected gastric cancer and D2 lymph node dissection.8 Although original trial failed to show a benefit with the addition of radiotherapy in the adjuvant setting, the data were re-analyzed with the 6 newly defined gastric cancer subtypes.
The investigators used NanoString technology on available RNA samples from the ARTIST trial to subdivide patients based on their expression signature. Interestingly, most subgroups reflected similar outcomes with both XP and XP/RT except group 3, which should yield a statistically significant benefit with the addition of radiotherapy (P = .03).
“This suggests that there may be a small subset of patients who benefit from radiation treatment in adjuvant setting,” Lee said.
Subtype 3 is characterized by a high number of copy number alterations, low methylation, moderate-to-high expression of mRNA, and a high level of HER2 expression. This is also the only subgroup that is characterized by a high response to radiation therapy.
To conclude, Lee reviewed characteristics of the 6 newly defined gastric cancer subtypes and noted that further research to validate the results are needed (TABLE).
TABLE. Summary of 6 Gastric Cancer Subtypes Defined by Ju-Seog Lee, PhD, et al.
References
- Lee J-S, et al. Clinically conserved subtypes from meta-analysis of genomic data from human gastric cancer. Presented at: 2022 International Gastric Cancer Congress; March 6-9, 2022; Houston, TX.
- Oh SC, Sohn BH, Cheong JH, et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat Commun. 2018;9(1):1777. doi:10.1038/s41467-018-04179-8
- Sohn BH, Shim JJ, Kim SB, et al. Inactivation of Hippo pathway is significantly associated with poor [rognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22(5):1256-1264. doi:10.1158/1078-0432.CCR-15-1447
- Sohn BH, Hwang JE, Jang HJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas Project. Clin Cancer Res. 2017;23(15):4441-4449. doi:10.1158/1078-0432.CCR-16-2211
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449-456. doi:10.1038/nm.3850
- Cheong JH, Yang HK, Kim H, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018;19(5):629-638. doi:10.1016/S1470-2045(18)30108-6
- Shin MK, Kim J, Kim D, et al. Long non-coding RNAs are significantly associated with prognosis and response to therapies in gastric cancer. Clin Transl Med. 2021;11(6):e421. doi:10.1002/ctm2.421
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268-273. doi:10.1200/JCO.2011.39.1953