Iodine-131 Tositumomab for Patients With Low-Grade or Transformed Low-Grade Non-Hodgkin’s Lymphoma: Complete Response Data
Tositumomab/iodine-131 tositumomab (Bexxar) is a radioimmunotherapeutic agent in development for patients with low-grade or transformed non-Hodgkin’s lymphoma (NHL). This analysis focuses
Tositumomab/iodine-131 tositumomab (Bexxar) is a radioimmunotherapeutic agent in development for patients with low-grade or transformed non-Hodgkin’s lymphoma (NHL). This analysis focuses on the complete response (CR) data from 269 evaluable patients who received tositumomab/iodine-131 tositumomab in phase I-III trials from 1990 to 1999.
A total of 42% of patients had received one to three prior chemotherapies and 33% of patients had received four prior chemotherapies. Baseline characteristics were as follows: 70% less than 60 years old, 56% male, 89% stage III or IV at protocol entry, 46% positive bone marrow involvement at study entry, and 32% bulky disease (> 500 g).
Patients received tositumomab/iodine-131 tositumomab as previously described (Blood 90:1259, 2000; J Clin Oncol 18:1316, 2000). A total of 88 patients (33%) had a CR; 74 (28%) had a confirmed CR (two assessments 4 weeks apart). The median follow-up was 1.5 years (range: 1 day to 8 years). The median duration of CR was 3.25 years, and that for confirmed CR was 5 years. The table below displays the subgroups that were significantly different (P < .05) via univariate analyses:
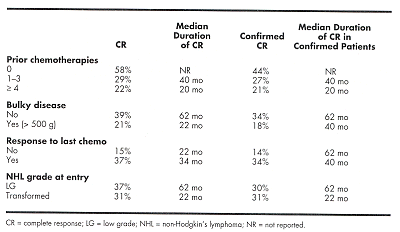
CONCLUSION: These results demonstrate that tositumomab/iodine-131 tositumomab produces a high CR rate in patients with low-grade or transformed low-grade NHL, and that these CRs are durable.
Click here to read Dr. Bruce Cheson's commentary on this abstract.